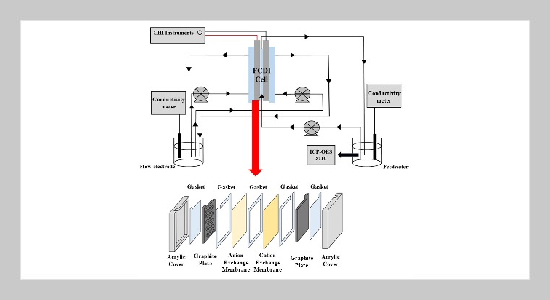
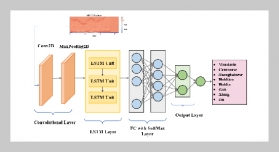
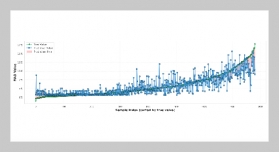
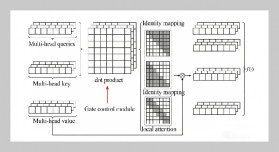
- [1] D. Cordell, A. Rosemarin, J. J. Schröder, and A. Smit, (2011) “Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options" Chemosphere 84(6): 747–758. DOI: 10.1016/j.chemosphere.2011.02.032.
- [2] T. Almeelbi and A. Bezbaruah. “Aqueous phosphate removal using nanoscale zero-valent iron”. In: Nanotechnology for Sustainable Development. Springer. 2014, 197–210. DOI: 10.1007/s11051-012-0900-y.
- [3] X. Yi, H. Zhong, M. Xie, and X. Wang, (2021) “A novel forward osmosis reactor assisted with microfiltration for deep thickening waste activated sludge: performance and implication" Water Research 195: 116998. DOI: 10.1016/j.waters.2021.116998.
- [4] J. Ren, Z. Zhu, Y. Qiu, F. Yu, J. Ma, and J. Zhao, (2021) “Magnetic field assisted adsorption of pollutants from an aqueous solution: A review" Journal of Hazardous Materials 408: 124846. DOI: 10.1016/j.jhazmat.2020.124846.
- [5] T. H. Boyer, A. Persaud, P. Banerjee, and P. Palomino, (2011) “Comparison of low-cost and engineered materials for phosphorus removal from organic-rich surface water" Water research 45(16): 4803–4814. DOI: 10.1016/j.waters.2011.06.020.
- [6] T. Tervahauta, R. D. Van Der Weijden, R. L. Flem ming, L. H. Leal, G. Zeeman, and C. J. Buisman, (2014) “Calcium phosphate granulation in anaerobic treat ment of black water: a new approach to phosphorus recovery" Water research 48: 632–642. DOI: 10.1016/j. waters.2013.10.012.
- [7] Y. Lei, E. Geraets, M. Saakes, R. D. van der Weijden, and C. J. Buisman, (2020) “Electrochemical removal of phosphate in the presence of calcium at low current density: precipitation or adsorption?" Water research 169: 115207. DOI: 10.1016/j.waters.2019.115207.
- [8] M. Zubrowska-Sudol and J. Walczak, (2015) “Enhancing combined biological nitrogen and phosphorus removal from wastewater by applying mechanically disintegrated excess sludge" Water Research 76: 10–18. DOI:10.1016/j.waters.2015.02.041.
- [9] J. Wilsenach, C. Schuurbiers, and M. Van Loosdrecht, (2007) “Phosphate and potassium recovery from source separated urine through struvite precipitation" Water research 41(2): 458–466. DOI: 10.1016/j.waters.2006.10.014.
- [10] M. A. Anderson, A. L. Cudero, and J. Palma, (2010) “Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete?" Electrochimica Acta 55(12): 3845–3856. DOI: 10.1016/j.electacta.2010.02.012.
- [11] S. Porada, R. Zhao, A. van der Wal, V. Presser, and P. M. Biesheuvel, (2013) “Review on the science and technology of water desalination by capacitive deionization" Progress in materials science 58(8): 1388–1442. DOI: 10.1016/j.pmatsci.2013.03.005.
- [12] M. E. Suss, S. Porada, X. Sun, P. M. Biesheuvel, J. Yoon, and V. Presser, (2015) “Water desalination via capacitive deionization: what is it and what can we expect from it?" Energy & Environmental Science 8(8): 2296–2319.
- [13] W. Xing, J. Liang, W. Tang, G. Zeng, X. Wang, X. Li, L. Jiang, Y. Luo, X. Li, N. Tang, et al., (2019) “Perchlorate removal from brackish water by capacitive deionization: Experimental and theoretical investigations" Chemical engineering journal 361: 209–218. DOI: 10.1016/j.cej.2018.12.074.
- [14] K. S. Lee, Y. Cho, K. Y. Choo, S. Yang, M. H. Han, and D. K. Kim, (2018) “Membrane-spacer assembly for f low-electrode capacitive deionization" Applied Surface Science 433: 437–442. DOI: 10.1016/j.apsusc.2017.10.021.
- [15] C. Zhang, D. He, J. Ma, W. Tang, and T. D. Waite, (2018) “Faradaic reactions in capacitive deionization (CDI)-problems and possibilities: A review" Water research 128: 314–330. DOI: 10.1016/j.waters.2017.10.024.
- [16] K. Tang, S. Yiacoumi, Y. Li, and C. Tsouris, (2018) “Enhanced water desalination by increasing the electro conductivity of carbon powders for high-performance f low-electrode capacitive deionization" ACS Sustain able Chemistry & Engineering 7(1): 1085–1094. DOI: 10.1021/acssuschemeng.8b04746.
- [17] J. Dykstra, S. Porada, A. Van Der Wal, and P. Biesheuvel, (2018) “Energy consumption in capacitive deionization–Constant current versus constant voltage operation" Water research 143: 367–375. DOI: 10.1016/j.waters.2018.06.034.
- [18] A. Ramachandran, D. I. Oyarzun, S. A. Hawks, M. Stadermann, and J. G. Santiago, (2019) “High water recovery and improved thermodynamic efficiency for capacitive deionization using variable flowrate operation" Water research 155: 76–85. DOI: 10.1016/j.waters.2019.02.007.
- [19] W. Tang, J. Liang, D. He, J. Gong, L. Tang, Z. Liu, D. Wang, and G. Zeng, (2019) “Various cell architectures of capacitive deionization: Recent advances and future trends" Water research 150: 225–251. DOI: 10.1016/j.waters.2018.11.064.
- [20] W. Xing, J. Liang, W. Tang, D. He, M. Yan, X. Wang, Y. Luo, N. Tang, and M. Huang, (2020) “Versatile applications of capacitive deionization (CDI)-based technologies" Desalination 482: 114390. DOI: 10.1016/j.desal.2020. 114390.
- [21] J. Jiang, D. I. Kim, P. Dorji, S. Phuntsho, S. Hong, and H. K. Shon, (2019) “Phosphorus removal mechanisms from domestic wastewater by membrane capacitive deionization and system optimization for enhanced phosphate removal" Process Safety and Environmental Protection 126: 44–52. DOI: 10.1016/j.psep.2019.04.005.
- [22] C. Zhang, J. Ma, L. Wu, J. Sun, L. Wang, T. Li, and T. D. Waite, (2021) “Flow electrode capacitive deionization (FCDI): recent developments, environmental applications, and future perspectives" Environmental Science &Technology 55(8): 4243–4267. DOI: 10.1021/acs.est.0c06552.
- [23] S.-i. Jeon, H.-r. Park, J.-g. Yeo, S. Yang, C. H. Cho, M. H. Han, and D. K. Kim, (2013) “Desalination via a new membrane capacitive deionization process utilizing flow-electrodes" Energy & Environmental Science 6(5): 1471–1475. DOI: 10.1039/c3ee24443a.
- [24] Y. Gendel, A. K. E. Rommerskirchen, O. David, and M. Wessling, (2014) “Batch mode and continuous desalination of water using flowing carbon deionization (FCDI) technology" Electrochemistry Communications 46: 152–156. DOI: 10.1016/j.elecom.2014.06.004.
- [25] G. Doornbusch, J. Dykstra, P. Biesheuvel, and M. Suss, (2016) “Fluidized bed electrodes with high carbon loading for water desalination by capacitive deionization" Journal of Materials Chemistry A 4(10): 3642–3647. DOI: 10.1039/c5ta10316a.
- [26] S. Yang, J. Choi, J.-g. Yeo, S.-i. Jeon, H.-r. Park, and D. K.Kim, (2016)“Flow-electrode capacitive deionization using an aqueous electrolyte with a high salt concentration" Environmental science & technology 50(11): 5892–5899. DOI: 10.1021/acs.est.5b04640.
- [27] P. Nativ, Y. Badash, and Y. Gendel, (2017) “New in sights into the mechanism of flow-electrode capacitive deionization" Electrochemistry Communications 76: 24–28. DOI: 10.1016/j.elecom.2017.01.008.
- [28] M. Wang, S. Hou, Y. Liu, X. Xu, T. Lu, R. Zhao, and L. Pan, (2016) “Capacitive neutralization deionization with flowelectrodes" Electrochimica Acta 216: 211–218. DOI: 10.1016/j.electacta.2016.09.026.
- [29] C. Zhang, J. Ma, and T. D. Waite, (2019) “Ammonia rich solution production from wastewaters using chemical free flow-electrode capacitive deionization" ACS Sustain able Chemistry & Engineering 7(7): 6480–6485. DOI: 10.1021/acssuschemeng.9b00314.
- [30] K. B. Hatzell, M. C. Hatzell, K. M. Cook, M. Boota, G. M. Housel, A. McBride, E. C. Kumbur, and Y. Gogotsi, (2015) “Effect of oxidation of carbon material on suspension electrodes for flow electrode capacitive deionization" Environmental science & technology 49(5): 3040–3047. DOI: 10.1021/es5055989.
- [31] A. Rommerskirchen, Y. Gendel, and M. Wessling, (2015) “Single module flow-electrode capacitive deioniza tion for continuous water desalination" Electrochemistry Communications 60: 34–37. DOI: 10.1016/j.elecom.2015.07.018.
- [32] H.-r. Park, J. Choi, S. Yang, S. J. Kwak, S.-i. Jeon, M. H. Han, and D. K. Kim, (2016) “Surface-modified spherical activated carbon for high carbon loading and its desalting performance in flow-electrode capacitive deionization" RSC advances 6(74): 69720–69727. DOI: 10.1039/C6RA02480G.
- [33] J. Ma, D. He, W. Tang, P. Kovalsky, C. He, C. Zhang, and T. D. Waite, (2016) “Development of redox-active f low electrodes for high-performance capacitive deionization" Environmental Science & Technology 50(24): 13495–13501. DOI: 10.1021/acs.est.6b03424.
- [34] S. Yang, S.-i. Jeon, H. Kim, J. Choi, J.-g. Yeo, H.-r. Park, and D. K. Kim, (2016) “Stack design and operation for scaling up the capacity of flow-electrode capacitive deionization technology" ACS Sustainable Chemistry & Engineering 4(8): 4174–4180. DOI: 10.1021/acssuschemeng.6b00689.
- [35] S. Yang, H.-r. Park, J. Yoo, H. Kim, J. Choi, M. H. Han, and D. K. Kim, (2017) “Plate-shaped graphite for improved performance of flow-electrode capacitive deionization" Journal of The Electrochemical Society 164(13): E480. DOI: 10.1149/2.1551713jes.
- [36] X. Xu, M. Wang, Y. Liu, T. Lu, and L. Pan, (2017) “Ultrahigh desalinization performance of asymmetric flow electrode capacitive deionization device with an improved operation voltage of 1.8 V" ACS Sustainable Chemistry & Engineering 5(1): 189–195. DOI: 10.1021/acssuschemeng.6b01212.
- [37] Y. Bian, X. Chen, L. Lu, P. Liang, and Z. J. Ren, (2019) “Concurrent nitrogen and phosphorus recovery using flow-electrode capacitive deionization" ACS Sustainable Chemistry & Engineering 7(8): 7844–7850. DOI: 10.1021/acssuschemeng.9b00065.
- [38] J. Zhang, L. Tang, W. Tang, Y. Zhong, K. Luo, M. Duan, W. Xing, and J. Liang, (2020) “Removal and recovery of phosphorus from low-strength wastewaters by f low-electrode capacitive deionization" Separation and Purification Technology 237: 116322. DOI: 10.1016/j.seppur.2019.116322.
- [39] C. Zhang, M. Wang, W. Xiao, J. Ma, J. Sun, H. Mo, and T. D. Waite, (2021) “Phosphate selective recovery by magnetic iron oxide impregnated carbon flow-electrode capacitive deionization (FCDI)" Water Research 189: 116653. DOI: 10.1016/j.waters.2020.116653.
- [40] Y. Zhan, F. Meng, Y. Lei, R. Zhao, J. Zhong, and X. Liu, (2011) “One-pot solvothermal synthesis of sandwich-like graphene nanosheets/Fe3O4 hybrid material and its microwave electromagnetic properties" Materials Letters 65(11): 1737–1740. DOI: 10.1016/j.matlet.2011.03.019.
- [41] Y.-b. Tang, Q. Liu, and F.-y. Chen, (2012) “Preparation and characterization of activated carbon from waste ramulusmori" Chemical Engineering Journal 203: 19–24. DOI: 10.1016/j.cej.2012.07.007.
- [42] C.-M. Hung, C.-W. Chen, Y.-Z. Jhuang, and C.-D. Dong, (2016) “Fe3O4 magnetic nanoparticles: characterization and performance exemplified by the degradation of methylene blue in the presence of persulfate" Journal of Advanced Oxidation Technologies 19(1): 43–51. DOI: 10.1515/jaots-2016-0105.
- [43] C.-L. Yeh, H.-C. Hsi, K.-C. Li, and C.-H. Hou, (2015) “Improved performance in capacitive deionization of activated carbon electrodes with a tunable mesopore and micropore ratio" Desalination 367: 60–68. DOI: 10.1016/j.desal.2015.03.035.