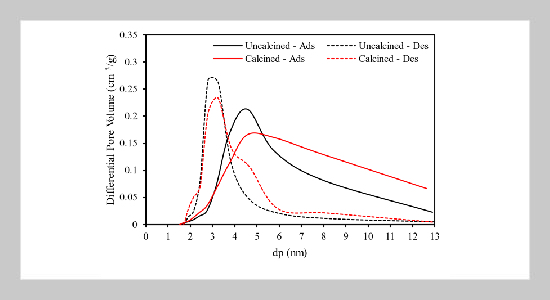
- [1] I. N. Meiyati, (2015) “Pemanfaatan Lumpur Geothermal (Geothermal Sludge) Untuk Pengganti Sebagian Semen Terhadap Kuat Tekan Mortar Sebagai Suplemen Bahan Ajar Mata Kuliah Teknologi Beton Program Studi Pen didikan Teknik Bangunan UNS" Indonesian Journal Of Civil Engineering Education UNS 15: 28. DOI: 10.20961/ijcee.v2i2.17936.
- [2] S. Sulardjaka, M. Rahman, and C. Wahyudianto, (2013) “Pengaruh Waktu Dan Temperatur Sinter Terhadap Densitas DanPorositas Komposit AluminiumYang Diperkuat Limbah Geothermal" Rotasi 15: 28–32. DOI: 10.14710/rotasi.15.4.28-32.
- [3] S. A. Jenie, A. Ghaisani, Y. P. Ningrum, A. Kristiani, F. Aulia, and H. T. Petrus. Preparation of silica nanopar ticles from geothermal sludge via sol-gel method. 2018. DOI: 10.1063/1.5064968.
- [4] C.Chircov, A. Spoial˘a, C. P˘aun, L. Cr˘aciun, D. Ficai, A. Ficai, E. Andronescu, and S , . C. Turculet, (2020) “Mesoporous silica platforms with potential applications in release and adsorption of active agents" Molecules 25(17): 3814. DOI: 10.3390/molecules25173814.
- [5] E. Yamamoto and K. Kuroda, (2018) “Preparation and controllability of mesoporous silica nanoparticles" The Enzymes 44: 1–10. DOI: 10.1016/bs.enz.2018.09.001.
- [6] D. ¸S. Karaman and H. Kettiger, (2018) “Silica based nanoparticles as drug delivery systems: Chances and challenges" Inorganic frameworks as smart nanomedicines: 1–40. DOI: 10.1016/B978-0-12 813661-4.00001-8.
- [7] C. Bharti, U. Nagaich, A. K. Pal, and N. Gulati, (2015) “Mesoporous silica nanoparticles in target drug delivery system: A review" International journal of pharmaceutical investigation 5: 1–40. DOI: 10.4103/2230 973x.160844.
- [8] A. Jabbari-Hichri, S. Bennici, and A. Auroux, (2016) “Effect of aluminum sulfate addition on the thermal storage performance of mesoporous SBA-15 and MCM-41 mate rials" Solar Energy Materials and Solar Cells 149: 232–241. DOI: 10.1016/j.solmat.2016.01.033.
- [9] J. Wang, H. Ge, and W. Bao, (2015) “Synthesis and characteristics of SBA-15 with thick pore wall and high hydrothermal stability" Materials letters 145: 312–315. DOI: 10.1016/j.matlet.2015.01.113.
- [10] H.-P. Lin, C.-Y. Tang, and C.-Y. Lin, (2002) “Detailed structural characterizations of sba-15 and mcm-41 mesoporous silicas on a high-resolution transmission electron microscope" Journal of the Chinese Chemical Society 49: 981–988. DOI: 10.1002/jccs.200200140.
- [11] R. Atluri. “Novel syntheses, structures and functions of mesoporous silica materials". (phdthesis). Acta Univer sitatis Upsaliensis, 2010.
- [12] N.I. Vazquez, Z. Gonzalez, B. Ferrari, and Y. Castro, (2017) “Synthesis of mesoporous silica nanoparticles by sol–gel as nanocontainer for future drug delivery applications" Boletín de la Sociedad Española de Cerámica y Vidrio 56: 139–145. DOI: 10.1016/j.bsecv.2017.03.002.
- [13] R. Rashid, F. Afroze, S. Ahmed, M. S. Miran, A. Bin, and H. Susan, (2019) “Control of the porosity and morphology of ordered mesoporous silica by varying calcina tion conditions" Materials Today 15: 546–554. DOI: 10.1016/j.matpr.2019.04.119.
- [14] N.Hao,X.Chen,K.W.Jayawardana,B.Wu,M.Sund horo, and M. Yan, (2015) “Shape control of mesoporous silica nanomaterials templated with dual cationic surfac tants and their antibacterial activities" Biomaterials sci ence 4: 87–91. DOI: 10.1039/C5BM00197H.
- [15] X.Fan, Y. Zhang, Y. Wang, F. Chen, and Y. Hui, (2022) “Ionic liquid modified mesoporous silica (SAB-15) as an effective polysulfide reservoir for high-performance lithium sulfur batteries" Advanced Powder Technology 33: 103530. DOI: 10.1016/j.apt.2022.103530.
- [16] S.-Y. Park and P. Pendleton, (2012) “Mesoporous silica SBA-15 for natural antimicrobial delivery" Powder technology 223: 77–82. DOI: 10.1016/j.powtec.2011.08.020.
- [17] P. Verma, Y. Kuwahara, K. Mori, R. Raja, and H. Ya mashita, (2020) “Functionalized mesoporous SBA-15 silica: recent trends and catalytic applications" Nanoscale 12: 11333–11363. DOI: 10.1039/D0NR00732C.
- [18] S. Porrang, N. Rahemi, S. Davaran, M. Mahdavi, and B. Hassanzadeh, (2021) “Preparation and in-vitro evaluation of mesoporous biogenic silica nanoparticles obtained from rice and wheat husk as a biocompatible carrier for anti-cancer drug delivery" European Journal of Pharmaceutical Sciences 163: 105866. DOI: 10.1016/j.ejps.2021.105866.
- [19] D.KavazandA.Vaseashta,(2019)“Synthesizing Nano Silica Nanoparticles from Barley Grain Waste: Effect of Temperature on Mechanical Properties." Polish Journal of Environmental Studies 28: DOI: http: //dx.doi.org/10.15244/pjoes/91078.
- [20] M. Sholeh, R. Rochmadi, H. Sulistyo, and B. Budhi janto, (2021) “Nanostructured silica from bagasse ash: the effect of synthesis temperature and pH on its properties" Journal of Sol-Gel Science and Technology 97: 126 137. DOI: https: //ir.lib.ugm.ac.id/id/eprint/17479.
- [21] A.A.AwalandM.W.Hussin,(1997)“Theeffectiveness of palm oil fuel ash in preventing expansion due to alkali silica reaction" Cement and Concrete Composites 19: 367–372. DOI: 10.1016/S0958-9465(97)00034-6.
- [22] S. Muljani, H. Setyawan, G. Wibawa, and A. Altway, (2014) “A facile method for the production of high-surface area mesoporous silica gels from geothermal sludge" Ad vanced Powder Technology 25: 1593–1599. DOI: http: //dx.doi.org/10.1016/j.apt.2014.05.012.
- [23] S. Silviana, E. A. P. P. Sagala, S. E. Sari, and C. T. M. Siagian, (2019) “Preparation of mesoporous silica derived from geothermal silica as precursor with a surfactant of cethyltrimethyl ammonium bromide" AIP Conference Proceedings 1: 020070. DOI: 10.1063/1.5141683.
- [24] H. Widiyandari, P. Pardoyob, J. Sartikab, O. Putraa, A. Purwantoc, and L. Ernawatid, (2021) “Synthesis of Mesoporous SiO2 Xerogel from Geothermal Sludge using Sulfuric Acid as Gelation Agent" International Journal of Engineering, Transactions A: Basics 34: 1569–1575. DOI: 10.5829/ije.2021.34.07a.02.
- [25] I. S. Cahyani, A. Prasetya, H. T. B. M. P, and C. W. Purnomo. Nanosilica from geothermal sludge using sol gel method with addition of CTAB surfactants. 2022. DOI: 10.1063/5.0072886.
- [26] A. M. Basso, B. P. Nicola, K. Bernardo-Gusmao, and S. B. Pergher, (2020) “Tunable effect of the calcination of the silanol groups of KIT-6 and SBA-15 mesoporous materials" Applied Sciences 10: 970. DOI: 10.3390/ app10030970.
- [27] M. Jannah, P. Taba, I. W. Sutapa, and Y. Hala. Adsorption of Ciprofloxacin from solution on mesoporous silica MCM-48: Kinetic study. 2021. DOI: 10.1063/5.0059487.
- [28] C. Carucci, N. Scalas, A. Porcheddu, M. Piludu, M. Monduzzi, and A. Salis, (2021) “Adsorption and re lease of sulfamethizole from mesoporous silica nanoparti cles functionalised with triethylenetetramine" Interna tional Journal of Molecular Sciences 22: 7665. DOI: 10.3390/ijms22147665.
- [29] V.Nairi, L. Medda, M. Monduzzi, and A.Salis, (2017) “Adsorption and release of ampicillin antibiotic from ordered mesoporous silica" Journal of colloid and inter face science 497: 217–225. DOI: 10.1016/j.jcis.2017.03.021.
- [30] N.Taebnia, D. Morshedi, S. Yaghmaei, F. Aliakbari, F. Rahimi, and A. Arpanaei, (2016) “Curcumin-loaded amine-functionalized mesoporous silica nanoparticles in hibit α-synuclein fibrillation and reduce its cytotoxicity associated effects" Langmuir 32: 13394–13402. DOI: 10. 1021/acs.langmuir.6b02935.
- [31] D. Patra, D. ¸S. Karaman, D. Desai, E. El Khoury, and J. M. Rosenholm, (2016) “Preparation of curcumin loaded mesoporous silica nanoparticles: determining polarizability inside the mesopores" Materials Research Bulletin 84: 267–272. DOI: 10.1016/j.materresbull.2016.08.012.
- [32] A. R. Adiatama, R. F. Susanti, W. Astuti, H. Petrus, and K. C. Wanta, (2022) “Synthesis and characteristic of nanosilica from geothermal sludge: Effect of surfactant" Metalurgi 37: 73. DOI: http: //dx.doi.org/10.14203/metalurgi.v37i2.637.
- [33] K. R, B. AK, P. S, F. M, B. D, and R.-D. A, (2016) “Cur cumin loaded mesoporous silica: An effective drug delivery system for cancer treatment" Biomaterials Science 1: 448–459. DOI: 10.1039/C5BM00552C.
- [34] D. D, G. S, A. L, and S. H, (2016) “Kajian Kinetika dan Isotherm Adsorpsi Limbah Cair Tapioka" Jurnal Rekayasa Kimia &Lingkungan1:10. DOI: 10.23955/ rkl.v11i1.4228.
- [35] A. Inyinbor, F. Adekola, and G. A. Olatunji, (2016) “Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp" Water Resources and Industry 15: 14 27. DOI: 10.1016/j.wri.2016.06.001.
- [36] J. Potgieter, S. Pearson, and C. Pardesi, (2018) “Kinetic and thermodynamic parameters for the adsorption of methylene blue using fly ash under batch, column, and heap leaching configurations" Coal combustion and gasification products 10: 23–33. DOI: http://dx.doi.org/10.4177/CCGP-D-17-00011.1.
- [37] T. R. Sahoo and B. Prelot. “Adsorption processes for the removal of contaminants from wastewater: the perspective role of nanomaterials and nanotechnology”. In: Nanomaterials for the detection and removal of wastewater pollutants. Elsiver, 2020, 161–222. DOI: 10.1016/B978-0-12-818489-9.00007-4.
- [38] B.An,(2020) “Cu(II) and As (V) adsorption kinetic characteristic of the multifunctional amino groups in chitosan" Processes 8: 1194. DOI: 10.3390/pr8091194.
- [39] N. M. Wulandari, L. Efiyanti, W. Trisunaryanti, H. S. Oktaviano, S. Bahri, Y. L. Ni’mah, and S. Larasati, (2021) “Effect of CTAB Ratio to the Characters of Mesoporous Silica Prepared from Rice Husk Ash in the Py rolysis of a-cellulose" Bulletin of Chemical Reaction Engineering & Catalysis 16: 632–640. DOI: 10.9767/bcrec.16.3.10828.632-640. [
- 40] M.Barczak, (2018) “Template removal from mesoporous silicas using different methods as a tool for adjusting their properties" New Journal of Chemistry 42: 4182–4191. DOI: 10.1039/C7NJ04642A.
- [41] M. A. Downing and P. K. Jain. “Mesoporous silica nanoparticles: synthesis, properties, and biomedical applications”. In: Nanoparticles for biomedical applica tions. Elsevier, 2020, 267–281. DOI: 10.1016/B978-0 12-816662-8.00016-3.
- [42] N. Rameli, K. Jumbri, R. Wahab, A. Ramli, and F. Huyop. Synthesis and characterization of mesoporous silica nanoparticles using ionic liquids as a template.
- [43] B. Purnawira, H. Purwaningsih, Y. Ervianto, V. Pratiwi, D. Susanti, R. Rochiem, and A. Purniawan. Synthesis and characterization of mesoporous silica nanoparticles (MSNp) MCM 41 from natural waste rice husk. 2019. DOI: 10.1088/1757-899X/541/1/012018.
- [44] T. TN, A. P. T. Van, P. L. ML, T. N. TP, and T. VM, (2013) “Synthesis of amorphous silica and sulfonic acid functionalized silica used as reinforced phase for polymer electrolyte membrane." Advances in Natural Sciences: Nanoscience and Nanotechnology 30: 045007. DOI: 10.1088/2043-6262/4/4/045007.
- [45] S. S. Jin L Kuo C hao, (2013) “Heterogeneous Catalysts for Biomass Conversion. New and Future Developments in Catalysis" New and Future Developments in Catalysis: 253–570. DOI: 10.1016/C2010-0-68566-X.
- [46] A. Bali´s and S. Zapotoczny, (2018) “Tailored synthesis of core-shell mesoporous silica particles—Optimization of dye sorption properties" Nanomaterials 8: 230. DOI: 10.3390/nano8040230.
- [47] S.Kim,M.-J. Stébé, J.-L. Blin, and A. Pasc, (2014) “pH controlled delivery of curcumin from a compartmentalized solid lipid nanoparticle@ mesostructured silica matrix" Journal of Materials Chemistry B 2: 7910–7917. DOI: 10.1039/C4TB01133C.
- [48] J. Goworek, A. Kierys, W. Gac, A. Borówka, and R. Kusak, (2009) “Thermal degradation of CTAB in as synthesized MCM-41" Journal of thermal analysis and calorimetry 96: 375–382. DOI: 10.1007/s10973 008-9055-6.
- [49] T.-H. Lo, Z.-Y. Wu, S.-Y. Chen, F.-Y. Meng, P.-T. Chou, C.-M. Wang, and H.-M. Lin, (2021) “Curcumin loaded mesoporous silica nanoparticles with dual-imaging and temperature control inhibits the infection of Zikavirus" Microporous and Mesoporous Materials 314: 110886. DOI: 10.1016/j.micromeso.2021.110886.
- [50] B. Bera and N. Das, (2019) “Synthesis of high surface area mesoporous silica SBA-15 for hydrogen storage ap plication" International Journal of Applied Ceramic Technology 16: 294–303. DOI: 10.1111/ijac.13082.
- [51] A. Romeiro, D. Freitas, M. Emília Azenha, M. Canle, and H. D. Burrows, (2017) “Effect of the calcination temperature on the photocatalytic efficiency of acidic sol gel synthesized TiO2 nanoparticles in the degradation of alprazolam" Photochemical & Photobiological Sci ences: 935–945. DOI: 10.1039/C6PP00447D.
- [52] Z. Zhang, A. Mayoral, and I. Melián-Cabrera, (2016) “Protocol optimization for the mild detemplation of mesoporous silica nanoparticles resulting in enhanced texture and colloidal stability" Microporous and Mesoporous Materials 220: 110–119. DOI: 10.1016/j.micromeso. 2015.08.026.
- [53] F. Kleitz, W. Schmidt, and F. Schüth, (2003) “Calcination behavior of different surfactant-templated mesostruc tured silica materials" Microporous and Mesoporous Materials 65: 1–29. DOI: 10.1016/S1387-1811(03) 00506-7.
- [54] R. Z. Y. ¸Sahin, (2021) “Understanding the Effect of Cal cination Process on the Mesoporous MCM-41 Material Morphology" Journal of the Turkish Chemical Society Section B: Chemical Engineering 4: 27–34. DOI: 10.1016/S1387-1811(03)00290-7.
- [55] Y. Chen, Y. Lu, R. J. Lee, and G. Xiang, (2020) “Nano encapsulated curcumin: and its potential for biomedical applications" International journal of nanomedicine 15: 3099–3120. DOI: 10.2147/IJN.S210320.
- [56] B. G. Trewyn, I. I. Slowing, S. Giri, H.-T. Chen, and V. S.-Y. Lin, (2007) “Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol–gel pro cess and applications in controlled release" Accounts of chemical research 40(9): 846–853. DOI: 10.1021/ ar600032u.
- [57] V. Candela-Noguera, P. Amorós, E. Aznar, M. D. Marcos, and R. Martínez-Máñez, (2024) “Systematic study of the implications of calcination and solvent ex traction of the surfactant in MCM-41-type mesoporous silica nanoparticles" Microporous and Mesoporous Materials 373: 113119.
- [58] A. H. Karim, A. A. Jalil, S. Triwahyono, S. Sidik, N. Kamarudin, R. Jusoh, N. Jusoh, and B. Hameed, (2012) “Amino modified mesostructured silica nanoparticles for efficient adsorption of methylene blue" Journal of Colloid and Interface Science: 307–314. DOI: 10.1016/j.jcis.2012.07.043.
- [59] B. Xing and J. Pignatello, (2005) “Sorption| organic chemicals" Encyclopedia of Soils in the Environ ment: 537–548. DOI: 10.1016/B0-12-348530-4/00555-5.