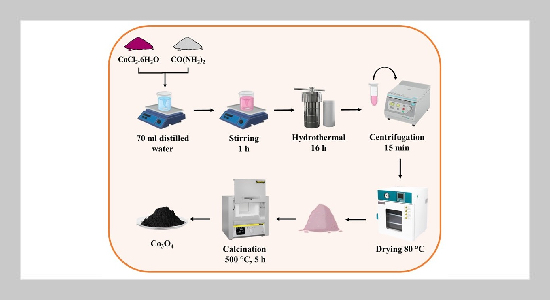
- [1] K.V.Jarnda, D.Wang,R.Anaman,V.E.Johnson,G.P. Roberts, P. S. Johnson, B. W. Jallawide Jr, T. Kai, P. Ding, et al., (2023) “Recent advances in electrochemical non-enzymatic glucose sensor for the detection of glucose in tears and saliva: A Review" Sensors and Actuators A: Physical 363: 114778. DOI: 10.1016/j.sna.2023.114778.
- [2] M. Thomas, (2022) “The clustering of cardiovascular, renal, adipo-metabolic eye and liver disease with type 2 diabetes" Metabolism 128: 154961. DOI: 10.1016/j.metabol.2021.154961.
- [3] H. Liang, Y. Luo, Y. Xiao, R. Chen, L. Wang, and Y. Song, (2024) “Ni/NiO/carbon derived from covalent organic frameworks for enzymatic-free electrochemical glucose sensor" Ceramics International 50(1): 977–984. DOI: 10.1016/j.ceramint.2023.10.188.
- [4] H.Imanzadeh, M. Amiri, and M. Nozari-Asbemarz, (2024) “A novel NiO/C@ rGO nanocomposite derived from Ni (gallate): A non-enzymatic electrochemical glu cose sensor" Microchemical Journal 199: 110106. DOI: 10.1016/j.microc.2024.110106.
- [5] S. Yadav, N. Rani, and K. Saini, (2024) “Fabrication of α-Fe2O3/rGO nanocomposite and its study for electro chemical glucose sensor and supercapacitor application" Diamond and Related Materials 142: 110707. DOI: 10.1016/j.diamond.2023.110707.
- [6] H.Miao, Z.Yang, J. Lv, and C. Kong, (2024) “Development of Cu2O-Cu-ZnO ternary nanocomposites for high performance photoelectrochemical non-enzymatic glucose sensor" Surfaces and Interfaces 46: 104195. DOI: 10.1016/j.surfin.2024.104195.
- [7] J.Zhang, Q.Xiong, and J.Xu,(2024)“Researchprogress in non-precious metal oxide/compound-based electrodes for non-enzymatic electrochemical glucose sensor applications" Materials Science in Semiconductor Processing 181: 108643. DOI: 10.1016/j.mssp.2024.108643.
- [8] S. K. Godlaveeti, S. K. Arla, A. El-marghany, A. R. Somala, V. K. N. Boya, R.R.Nagireddy, and S.W.Joo, (2024) “Nanostructured 3-D nano-bricks shaped CuO 0-D particle shaped Co3O4 composite for superior photo electrochemical water splitting" International Journal of Hydrogen Energy 58: 1000–1008. DOI: 10.1016/j.ijhydene.2024.01.177.
- [9] R. Subagyo, A. Yudhowijoyo, N. A. Sholeha, S. S. Hutagalung, D. Prasetyoko, M. D. Birowosuto, A. Arramel, J. Jiang, and Y. Kusumawati, (2023) “Re cent advances of modification effect in Co3O4-based cata lyst towards highly efficient photocatalysis" Journal of Colloid and Interface Science 650: 1550–1590. DOI: 10.1016/j.jcis.2023.07.117.
- [10] S. L. Jadhav, A. L. Jadhav, P. B. Sarawade, B. K. Man dlekar, and A. V. Kadam, (2024) “Mo-doped porous Co3O4 nanoflakes as an electrode with the enhanced ca pacitive contribution for asymmetric supercapacitor ap plication" Journal of Energy Storage 82: 110540. DOI: 10.1016/j.est.2024.110540.
- [11] P.-P. Chen, H.-B. Guan, B.-H. Zhang, J.-T. Lei, Z.-A. Li, Y.-L. Hou, J.-Z. Chen, and D.-L. Zhao, (2024) “Graphene encapsulated NiO-Co3O4 open-ended hol low nanocube anode with enhanced performance for lithium/sodium ion batteries" Journal of Alloys and Compounds979: 173479. DOI: 10.1016/j.jallcom.2024. 173479.
- [12] J. Yi, X. Li, S. Lv, J. Zhu, Y. Zhang, X. Li, and Y. Cong, (2023) “MOF-derived CeO2/Co3O4–Fe2O3@ CC nanocomposites as highly sensitive electrochemical sensor for bisphenol A detection" Chemosphere 336: 139249. DOI: 10.1016/j.chemosphere.2023.139249.
- [13] M. Shandilya, R. Rai, and J. Singh, (2016) “Hy drothermal technology for smart materials" Advances in Applied Ceramics 115(6): 354–376. DOI: 10.1080/17436753.2016.1157131.
- [14] N.K. Yetim, (2021) “Hydrothermal synthesis of Co3O4 with different morphology: Investigation of magnetic and electrochemical properties" Journal of Molecular Structure 1226: 129414. DOI: 10.1016/j.molstruc.2020.129414.
- [15] X. Lin, Y. Wang, M. Zou, T. Lan, and Y. Ni, (2019) “Electrochemical non-enzymatic glucose sensors based on nano-composite of Co3O4 and multiwalled carbon nanotube" Chinese Chemical Letters 30(6): 1157–1160. DOI: 10.1016/j.cclet.2019.04.009.
- [16] V. Archana, Y. Xia, R. Fang, and G. Gnana Kumar, (2019) “Hierarchical CuO/NiO-carbon nanocomposite derived from metal organic framework on cello tape for the flexible and high performance nonenzymatic electro chemical glucose sensors" ACS Sustainable Chemistry & Engineering 7(7): 6707–6719. DOI: 10.1021/acssuschemeng.8b05980.
- [17] A. Mustafa, I. A. Alsafari, H. Somaily, S. Yousaf, M. I. Din, J. Rahman, M. Shahid, M. Ashraf, and M.F.Warsi,(2023)“Fabrication, characterization of NiO Co3O4/rGO based nanohybrid and application in the de velopment of non-enzymatic glucose sensor" Physica B: CondensedMatter648:414404. DOI:10.1016/j.physb.2022.414404.
- [18] B. A. Hussein, A. A. Tsegaye, G. Shifera, and A. M. Taddesse, (2023) “A sensitive non-enzymatic electro chemical glucose sensor based on a ZnO/Co3O4/reduced graphene oxide nanocomposite" Sensors & Diagnostics 2(2): 347–360. DOI: 10.1039/D2SD00183G.
- [19] I. Liaqat, N. Iqbal, M. Aslam, M. Nasir, A. Hayat, D. X. Han, L. Niu, and M. H. Nawaz, (2020) “Co3O4 nanocubes decorated single-walled carbon nanotubes for efficient electrochemical non-enzymatic glucose sensing" SN Applied Sciences 2: 1–12. DOI: 10.1007/s42452 020-03531-2.
- [20] T.Yuwen, D.Shu, H.Zou, X.Yang, S.Wang, S.Zhang, Q. Liu, X. Wang, G. Wang, Y. Zhang, et al., (2023) “Carbon nanotubes: a powerful bridge for conductivity and flexibility in electrochemical glucose sensors" Journal of Nanobiotechnology 21(1): 320. DOI: 10.1186/s12951 023-02088-7.
- [21] Y. Qiu and Y. Wang, (2022) “Controllable synthesis of porous Co3O4 nanorods and their ethanol-sensing performance" Ceramics International 48(20): 29659–29668. DOI: 10.1016/j.ceramint.2022.06.221.
- [22] X. Pan, F. Ji, Q. Xia, X. Chen, H. Pan, S. N. Khisro, S. Luo, M. Chen, and Y. Zhang, (2018) “High performance supercapacitors based on superior Co3O4 nanorods electrode for integrated energy harvesting storage system" Electrochimica Acta 282: 905–912. DOI: h10.1016/j.electacta.2018.06.127.
- [23] J. Zhang, Y. Sun, X. Li, and J. Xu, (2019) “Fabrication of porous NiMn2O4 nanosheet arrays on nickel foam as an advanced sensor material for non-enzymatic glucose detection" Scientific reports 9(1): 18121. DOI: 10.1038/s41598-019-54746-2.
- [24] J. Salamon, A. Simi, H. J. Prabu, A. F. Sahayaraj, I. Johnson, V. Snowlin, R. Gopi, and A. J. S. Kennedy, (2024) “Synthesis and characterization of graphene-doped Co3O4 nanotubes electrode material for supercapacitor application" Journal of Inorganic and Organometal licPolymers and Materials 34(6): 2555–2571. DOI: 10.1007/s10904-023-02995-0.
- [25] S. A. Makhlouf, Z. H. Bakr, K. I. Aly, and M. Moustafa, (2013) “Structural, electrical and optical properties of Co3O4 nanoparticles" Superlattices and Microstructures 64: 107–117. DOI: 10.1016/j.spmi.2013.09.023.
- [26] A.Diallo ,A.Beye, T.B.Doyle, E.Park, and M.Maaza, (2015) “Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: physical properties" Green Chemistry Letters and Reviews 8(3-4): 30–36. DOI: 10.1080/17518253.2015.1082646.
- [27] A. Abdallah and R. Awad, (2020) “Study of the struc tural and physical properties of Co3O4 nanoparticles synthesized by co-precipitation method" Journal of Superconductivity and Novel Magnetism 33(5): 1395 1404. DOI: 10.1007/s10948-019-05296-1.
- [28] D. D. M.Prabaharan, K. Sadaiyandi, M. Mahendran, and S. Sagadevan, (2017) “Precipitation method and characterization of cobalt oxide nanoparticles" Applied Physics A 123: 1–6. DOI: 10.1007/s00339-017-0786-8.
- [29] Y. Ding, Y. Wang, L. Su, M. Bellagamba, H. Zhang, and Y. Lei, (2010) “Electrospun Co3O4 nanofibers for sensitive and selective glucose detection" Biosensors and Bioelectronics 26(2): 542–548. DOI: 10.1016/j.bios.2010.07.050.
- [30] L. Yang, J. Zhang, M. Lv, Y. Ruan, X. Weng, and J. Feng, (2022) “Dual-function glucose and hydrogen per oxide sensors based on Copper-embedded porous carbon composites" Journal of Electroanalytical Chemistry 924: 116881. DOI: 10.1016/j.jelechem.2022.116881.
- [31] D. D. Upadhyay, S. Singh, K. B. Singh, N. Gau tam, S. Shrivastava, and G. Pandey, (2023) “Biogeni cally produced Co3O4 nanoparticles and their application as micronutrient and antimicrobial agent foragro environmental sustainability" Inorganic Chemistry Communications 155: 110957. DOI: 10.1016/j.inoche.2023.110957.
- [32] A. Deshpande and S. G. Bansod, (2024) “Effect of sintering temperature on sol–gel synthesized NASICON type Li1.3Al0.3Ti1.7(PO4)3 ceramic solid electrolyte" Journal of Materials Science: Materials in Electronics 35(2): 138. DOI: 10.1007/s10854-023-11766-z.
- [33] J. Zhang, J. Feng, Y. Tian, Y. Wu, X. Liu, and Q. He, (2021) “Ultrasensitive electrochemical determination of tyrosine based on the α-Fe2O3@Co3O4-NRGO modified electrode" Microchemical Journal 171: 106867. DOI: 10.1016/j.microc.2021.106867.
- [34] K. Yang, S. Cheng, Z. Yao, S. Li, and Y. Yang, (2024) “Dumbbell shaped nanocomposite Co3O4/CeO2 derived from metal-organic frameworks (MOFs) as an excellent non-enzymatic glucose sensor" Solid State Sciences 150: 107498. DOI: 10.1016/j.solidstatesciences.2024.107498.
- [35] N.Elgrishi, K.J.Rountree, B.D.McCarthy, E.S.Roun tree, T. T. Eisenhart, and J. L. Dempsey, (2018) “A practical beginner’s guide to cyclic voltammetry" Journal of chemical education 95(2): 197–206. DOI: 10. 1021/acs.jchemed.7b00361.
- [36] Q. Dong, X. Wang, W. S. Willis, D. Song, Y. Huang, J. Zhao, B. Li, and Y. Lei, (2019) “Nitrogen-doped hollow Co3O4 nanofibers for both solid-state pH sensing and improved non-enzymatic glucose sensing" Electroanalysis 31(4): 678–687. DOI: 10.1002/elan.201800741.
- [37] J. Zhao, C. Zheng, J. Gao, J. Gui, L. Deng, Y. Wang, and R. Xu, (2021) “Co3O4 nanoparticles embedded in laser-induced graphene for a flexible and highly sensitive enzyme-free glucose biosensor" Sensors and Actuators B: Chemical 347: 130653. DOI: 10.1016/j.snb.2021.130653.
- [38] S. Ramesh, K. Karuppasamy, Y. Haldorai, A. Sivasamy, H.-S. Kim, and H. S. Kim, (2021) “Hexago nal nanostructured cobalt oxide@ nitrogen doped multi walled carbon nanotubes/polypyyrole composite for super capacitor and electrochemical glucose sensor" Colloids and Surfaces B: Biointerfaces 205: 111840. DOI: 10.1016/j.colsurfb.2021.111840.
- [39] C. Li, J. Xiong, C. Zheng, and J. Zhao, (2022) “Screen printing preparation of high-performance nonenzymatic glucose sensors based on Co3O4 nanoparticles-embedded N-doped laser-induced graphene" ACS Applied Nano Materials 5(11): 16655–16663. DOI: 10.1021/acsanm. 2c03694.
- [40] P. Balla, A. Sinha, L. Wu, X. Lu, D. Tan, J. Chen, et al., (2019) “Co3O4 nanoparticles supported mesoporous car bon framework interface for glucose biosensing" Talanta 203: 112–121. DOI: 10.1016/j.talanta.2019.05.056.
- [41] M.Kang, H.Zhou, N.Zhao, andB.Lv,(2020)“Porous Co3O4 nanoplates as an efficient electromaterial for non enzymatic glucose sensing" Cryst Eng Comm 22(1): 35 43. DOI: 10.1039/C9CE01396B.
- [42] M.A.Yassin, B. K. Shrestha, R. Ahmad, S. Shrestha, C. H.Park,andC.S.Kim,(2019)“Exfoliatednanosheets of Co3O4 webbed with polyaniline nanofibers: A novel composite electrode material for enzymeless glucose sens ing application" Journal of Industrial and Engineer ing Chemistry 73: 106–117. DOI: 10.1016/j.jiec.2019. 01.011.
- [43] S.-J. Kang and J. J. Pak, (2022) “Synthesis of Laser Induced Cobalt Oxide for Non-Enzymatic Electro chemical Glucose Sensors" ChemElectroChem 9(16): e202200328. DOI: 10.1002/celc.202200328.
- [44] M. Hilal, W. Xie, and W. Yang, (2022) “Straw-sheaf like Co3O4 for preparation of an electrochemical non enzymatic glucose sensor" Microchimica Acta 189(9): 364. DOI: 10.1007/s00604-022-05453-9.
- [45] L. Yan, D. Chu, X.-Q. Chu, D. Ge, and X. Chen, (2022) “Co/CoO nanoparticles armored by N-doped nanoporous carbon polyhedrons towards glucose oxidation in high performance non-enzymatic sensors" New Journal of Chemistry 46(31): 15071–15079. DOI: 10.1039/D2NJ02490J.
- [46] A. Mohammadpour-Haratbar, B. Mosallanejad, Y. Zare, K. Y. Rhee, and S.-J. Park, (2022) “Co3O4 nanoparticles embedded in electrospun carbon nanofibers as free-standing nanocomposite electrodes as highly sensitive enzyme-free glucose biosensors" Reviews on Advanced Materials Science 61(1): 744–755. DOI: 10.1515/rams-2022-0251.
- [47] F. Liaqat, I. ul Haq, F. Saira, and S. Qaisar, (2023) “Development of glucose sensor based on cobalt and nickel doped ceria nanostructures" Materials Science and Engineering: B 289: 116231. DOI: 10.1016/j.mseb.2022.116231.
- [48] G. Wang, L. Li, X. Han, W. Gao, and C. Wang, (2024) “Nickel foam-decorated hollow-Co3O4/GO nanocompos ites: A highly sensitive non-enzymatic electrochemical sensor for glucose detection" Microchemical Journal 206: 111398. DOI: 10.1016/j.microc.2024.111398.
- [49] J. H. Han, H. W. Kang, W. Lee, et al., (2019) “Ultra-sensitive non-enzymatic amperometric glucose sensors based on silver nanowire/graphene hybrid three dimensional nanostructures" Results in Physics 15: 102761. DOI: 10.1016/j.rinp.2019.102761.