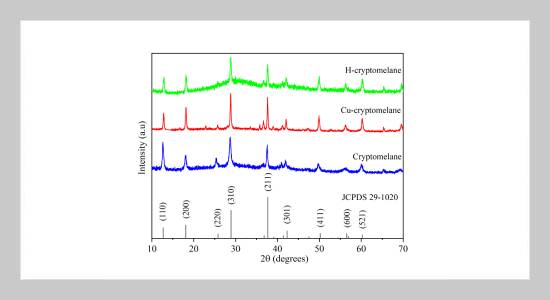
- [1] K.-H. Cohr and J. Stokholm, (1979) “Toluene: A toxicologic review" Scandinavian Journal of Work, Environment &Health 5(2): 71–90. DOI: 10.5271/sjweh.2664.
- [2] T.-T. Win-Shwe and H. Fujimaki, (2010) “Neurotoxicity of toluene" Toxicology Letters 198(2): 93–99. DOI: 10.1016/j.toxlet.2010.06.022.
- [3] E. A. Anigilaje, Z. A. Nasir, and C. Walton, (2024) “Exposure to benzene, toluene, ethylbenzene, and xylene (BTEX) at Nigeria’s petrol stations: a review of current status, challenges and future directions" Frontiers in Public Health 12: 1295758. DOI: 10.3389/fpubh.2024. 1295758.
- [4] J. H. Hannigan and S. E. Bowen, (2010) “Reproductive Toxicology and Teratology of Abused Toluene" Systems Biology in Reproductive Medicine 56(2): 184–200. DOI: 10.3109/19396360903377195.
- [5] K. Vellingiri, P. Kumar, A. Deep, and K.-H. Kim, (2017) “Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure" Chemical Engineering Journal 307: 1116–1126. DOI: 10.1016/j.cej.2016.09.012.
- [6] L. N. Q. Tu, N. V. H. Nhan, N. Van Dung, N. T. An, and N. Q. Long, (2019) “Enhanced photocatalytic performance and moisture tolerance of nano-sized Me/TiO2 zeolite Y (Me= Au, Pd) for gaseous toluene removal: activity and mechanistic investigation" Journal of Nanoparticle Research 21: 1–19. DOI: 10.1007/s11051-019-4642-y.
- [7] X. Zhang, J. Zhao, Z. Song, W. Liu, H. Zhao, M. Zhao, Y. Xing, Z. Ma, and H. Du, (2020) “The catalytic oxidation performance of toluene over the Ce-Mn-Ox catalysts: Effect of synthetic routes" Journal of Colloid and Interface Science 562: 170–181. DOI: 10.1016/j.jcis.2019.12.029.
- [8] S. Hoseini, N. Rahemi, S. Allahyari, and M. Tasbihi, (2019) “Application of plasma technology in the removal of volatile organic compounds (BTX) using manganese oxide nano-catalysts synthesized from spent batteries" Journal of Cleaner Production 232: 1134–1147. DOI: 10.1016/j.jclepro.2019.05.227.
- [9] S. L. Brock, N. Duan, Z. R. Tian, O. Giraldo, H. Zhou, and S. L. Suib, (1998) “A Review of Porous Manganese Oxide Materials" ChemistryofMaterials10(10): 2619 2628. DOI: 10.1021/cm980227h.
- [10] T.-M. Tran-Thuy, T.-P. Tran, and D. Van Nguyen, (2023) “Cobalt-doped cryptomelane: surface-tailored oxy gen defects and efficiently catalytic ozonation of p Nitrophenol" Topics in Catalysis 66(1): 289–296. DOI: 10.1007/s11244-022-01773-5.
- [11] K. Li, J. Chen, Y. Peng, W. Lin, T. Yan, and J. Li, (2017) “The relationship between surface open cells of α-MnO2 and CO oxidation ability from a surface point of view" Journal of Materials ChemistryA5(39):20911 20921. DOI: 10.1039/C7TA04624C.
- [12] V. P. Santos, M. F. R. Pereira, J. J. M. Órfão, and J. L. Figueiredo, (2009) “Catalytic oxidation of ethyl acetate over a cesium modified cryptomelane catalyst" Applied Catalysis B: Environmental 88(3-4): 550–556. DOI: 10.1016/j.apcatb.2008.10.006.
- [13] B. A. Cymes, C. B. Almquist, and M. P. S. Krekeler, (2020) “Europium-doped cryptomelane: Multi-pathway synthesis, characterization, and evaluation for the gas phase catalytic oxidation of ethanol" Applied Catalysis A: General 589: 117310. DOI: 10.1016/j.apcata.2019. 117310.
- [14] A. Davó-Quiñonero, M. Navlani-García, D. Lozano-Castelló, and A. Bueno-López, (2016) “CuO/cryptomelane catalyst for preferential oxidation of COinthe presence of H2: deactivation and regeneration" Catalysis Science & Technology 6(14): 5684–5692. DOI: 10.1039/c6cy00329j.
- [15] O. S. G. P. Soares, A. M. Fonseca, P. Parpot, J. J. M. Órfão, M. F. R. Pereira, and I. C. Neves, (2018) “Ox idation of volatile organic compounds by highly efficient metal zeolite catalysts" ChemCatChem 10(17): 3754–3760. DOI: 10.1002/cctc.201800524.
- [16] M. Romero-Sáez, D. Divakar, A. Aranzabal, J. R. González-Velasco, and J. A. González-Marcos, (2016) “Catalytic oxidation of trichloroethylene over Fe-ZSM-5: Influence of the preparation method on the iron species and the catalytic behavior" Applied Catalysis B: Envi ronmental 180: 210–218. DOI: 10.1016/j.apcatb.2015.06.027.
- [17] X. Li, Y. Wang, D. Chen, N. Li, Q. Xu, H. Li, J. He, and J. Lu, (2023) “A highly dispersed Pt/copper modified MnO2catalyst for the complete oxidation of volatile organic compounds: the effect of oxygen species on the catalytic mechanism" Green Energy & Environment 8(2): 538–547. DOI: 10.1016/j.gee.2021.07.004.
- [18] Y. Liu, W. Zong, H. Zhou, D. Wang, R. Cao, J. Zhan, L. Liu, and B. W.-L. Jang, (2018) “Tuning the interlayer cations of birnessite-type MnO2 to enhance its oxidation ability for gaseous benzene with water resistance" Catalysis Science & Technology 8(20): 5344–5358. DOI: 10.1039/C8CY01147H.
- [19] T.-M. Tran-Thuy, L.-D. Nguyen, H.-H. Lam, D. V. Nguyen, and T. Dang-Bao, (2020) “Tuning surfactant templates of nanorod-like cryptomelane synthesis towards vapor-phase selective oxidation of benzyl alcohol" Materials Letters 277: 128333. DOI: 10.1016/j.matlet.2020.128333.
- [20] L. Wang, K. Zhang, Z. Hu, W. Duan, F. Cheng, and J. Chen, (2014) “Porous CuO nanowires as the anode of rechargeable Na-ion batteries" Nano Research 7: 199 208. DOI: 10.1007/s12274-013-0387-6.
- [21] D. Zhou, X. Pu, Z. Jiao, and W. Li, (2022) “Controlled morphological synthesis of temperature-dependent CuO nanostructures and their effect on photocatalytic performance" Materials Research Express 9(9): 095501. DOI: 10.1088/2053-1591/ac8bc6.
- [22] G. H. Du and G. Van Tendeloo, (2004) “Cu(OH)2 nanowires, CuO nanowires and CuO nanobelts" Chemical Physics Letters 393(1-3): 64–69. DOI: 10.1016/j.cplett.2004.06.017.
- [23] W.Y. Hernández, M. A. Centeno, F. Romero-Sarria, S. Ivanova, M. Montes, and J. A. Odriozola, (2010) “Modified cryptomelane-type manganese dioxide nanomaterials for preferential oxidation of CO in the presence of hydrogen" Catalysis Today 157(1-4): 160–165. DOI: 10.1016/j.cattod.2010.03.010.
- [24] N.J. Mazumdar, G. Deshmukh, A. Rovea, P. Kumar, M. Arredondo-Arechavala, and H. Manyar, (2022) “Insights into selective hydrogenation of levulinic acid using copper on manganese oxide octahedral molecular sieves" Royal Society Open Science 9(7): 220078. DOI: 10.1098/rsos.220078.
- [25] A. Xie, Y. Tao, X. Jin, P. Gu, X. Huang, X. Zhou, S. Luo, C. Yao, and X. Li, (2019) “A β-Fe2O3-modified nanoflower-MnO2/attapulgite catalyst for low temperature SCR of NOx with NH3" New Journal of Chemistry 43(6): 2490–2500. DOI: 10.1039/C8NJ04524K.
- [26] Z. Xiao, J. Yang, R. Ren, J. Li, N. Wang, and W. Chu, (2020) “Facile synthesis of homogeneous hollow micro sphere Cu–Mn based catalysts for catalytic oxidation of toluene" Chemosphere 247: 125812. DOI: 10.1016/j.chemosphere.2020.125812.
- [27] M. Sun, B. Zhang, H. Liu, B. He, F. Ye, L. Yu, C. Sun, and H. Wen, (2017) “The effect of acid/alkali treatment on the catalytic combustion activity of manganese oxide octahedral molecular sieves" RSC advances 7(7): 3958–3965. DOI: 10.1039/C6RA27700D.
- [28] R. Kumar, S. Sithambaram, and S. L. Suib, (2009) “Cyclohexane oxidation catalyzed by manganese oxide octahedral molecular sieves—Effect of acidity of the catalyst" Journal of Catalysis 262(2): 304–313. DOI: 10.1016/j.jcat.2009.01.007.
- [29] S. I. Suárez-Vázquez, S. Gil, J. M. García-Vargas, A. Cruz-López, and A. Giroir-Fendler, (2018) “Catalytic oxidation of toluene by SrTi1-XBXO3 (B= Cu and Mn) with dendritic morphology synthesized by one pot hydrothermal route" Applied Catalysis B: Environmental 223: 201–208. DOI: 10.1016/j.apcatb.2017.04.042.
- [30] Z. Wang, L. Zhang, J. Ji, Y. Wu, Y. Cai, X. Yao, X. Gu, Y. Xiong, H. Wan, L. Dong, and Y.-W. Chen, (2022) “Catalytic enhancement of small sizes of CeO2 additives on Ir/Al2O3 for toluene oxidation" Applied Surface Science 571: 151200. DOI: 10.1016/j.apsusc.2021.151200.