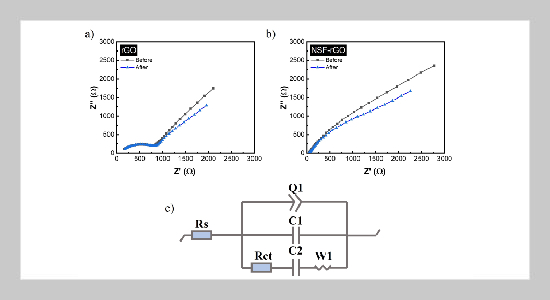
- [1] F. Ahmad, M. Khalid, and B. K. Panigrahi, (2021) “Development in energy storage system for electric transportation: A comprehensive review" Journal of Energy Storage 43: 103153. DOI: https://doi.org/10.1016/j.est.2021.103153.
- [2] T. M. Gur, (2018) “Review of electrical energy stor age technologies, materials and systems: challenges and prospects for large-scale grid storage" Energy & Environmental Science 11(10): 2696–2767. DOI: 10.1039/C8EE01419A.
- [3] S. K. Vineeth, M. Tebyetekerwa, H. Liu, C. B. Soni, Sungjemmenla, X. S. Zhao, and V. Kumar, (2022) “Progress in the development of solid-state electrolytes for reversible room-temperature sodium–sulfur batteries" Materials Advances 3(16): 6415–6440. DOI: 10.1039/D2MA00428C.
- [4] A.Y. S. Eng, C. B. Soni, Y. Lum, E. Khoo, Z. Yao, S. K. Vineeth, V. Kumar, J. Lu, C. S. Johnson, C. Wolverton, and Z. W. Seh, (2025) “Theory-guided experimental de sign in battery materials research" Science Advances 8(19): eabm2422. DOI: 10.1126/sciadv.abm2422.
- [5] J. F. M. J. Prasanth Raghavan, Akhila Das. “Metal Ion, Hybrid, and Metal-Air Batteries”. In: Advanced Technologies for Rechargeable Batteries. 1st. Boca Raton: CRC Press, 2024, 396. DOI: https://doi.org/10.1201/9781003310174.
- [6] S. K. Vineeth, A. Babu, A. Das, A. Pullanchiyodan, N. Gupta, V. Pillai, and P. Raghavan. “Metal Air Battery Future of Hybrid or Electric Vehicles (HEV or EV)”. In: Advanced Technologies for Rechargeable Batteries. 1st. Boca Raton: CRC Press, 2024, 396. DOI: https://doi.org/10.1201/9781003310174.
- [7] S. K. Vineeth, P. Sreeram, A. Vlad, R. Joy, P. Raghavan, and A. Pullanchiyodan. “7- Polymer blend nanocomposite electrolytes for advanced energy storage ap plications”. In: Micro and Nano Technologies. Ed. by S. Thomas, A. R. Ajitha, and M. B. T. .-. P. B. N. f. E. S. A. Jaroszewski. Elsevier, 2023, 203–238. DOI: https://doi.org/10.1016/B978-0-323-99549-8.00016-9.
- [8] N. S. Jishnu, S. K. Vineeth, A. Das, N. T. M. Balakrish nan, A. P. Thomas, M. J. Jabeen Fatima, J.-H. Ahn, and R. Prasanth. “Electrospun PVdF and PVdF-co-HFP Based Blend Polymer Electrolytes for Lithium Ion Batteries BT- Electrospinning for Advanced Energy Storage Applications”. In: ed. by N. T. M. Balakrish nan and R. Prasanth. Singapore: Springer Singapore, 2021, 201–234. DOI: 10.1007/978-981-15-8844-0_8.
- [9] C. Liu, X. Yan, F. Hu, G. Gao, G. Wu, and X. Yang, (2018) “Toward Superior Capacitive Energy Storage: Recent Advances in Pore Engineering for Dense Electrodes" Advanced Materials 30(17): 1705713. DOI: https://doi.org/10.1002/adma.201705713.
- [10] R. Dubey and V. Guruviah, (2019) “Review of carbon based electrode materials for supercapacitor energy storage" Ionics 25(4): 1419–1445. DOI: 10.1007/s11581-019-02874-0.
- [11] A. Borenstein, O. Hanna, R. Attias, S. Luski, T. Brousse, and D. Aurbach, (2017) “Carbon-based composite materials for supercapacitor electrodes: A review" Journal of Materials Chemistry A 5(25): 12653 12672. DOI: 10.1039/c7ta00863e.
- [12] W. Hooch Antink, Y. Choi, K.-d. Seong, J. M. Kim, and Y. Piao, (2018) “Recent Progress in Porous Graphene and Reduced Graphene Oxide-Based Nanomaterials for Electrochemical Energy Storage Devices" Advanced Materials Interfaces 5(5): 1701212. DOI: https://doi.org/10.1002/admi.201701212.
- [13] P. Zhang, Z. Li, S. Zhang, and G. Shao, (2018) “Recent Advances in Effective Reduction of Graphene Oxide for Highly Improved Performance Toward Electrochemical Energy Storage" Energy & Environmental Materials 1(1): 5–12. DOI: https://doi.org/10.1002/eem2.12001.
- [14] E. Budi Nursanto, A. Nugroho, S.-A. Hong, S. Kim, K. Yoon Chung, and J. Kim, (2011) “Facile synthesis of reduced graphene oxide in supercritical alcohols and its lithium storage capacity" Green Chemistry 13(10): 2714–2718. DOI: 10.1039/c1gc15678k.
- [15] M. Seo, D. Yoon, K. S. Hwang, J. W. Kang, and J. Kim, (2013) “Supercritical alcohols as solvents and reducing agents for the synthesis of reduced graphene oxide" Car bon 64: 207–218. DOI: https://doi.org/10.1016/j.carbon.2013.07.053.
- [16] M. Ghorbani, H. Abdizadeh, and M. Golobostanfard, (2015) “Reduction of Graphene Oxide via Modified Hydrothermal Method" Procedia Materials Science 11(2009): 326–330. DOI: 10.1016/j.mspro.2015.11.104.
- [17] Z. Y. Sui, Y. N. Meng, P. W. Xiao, Z. Q. Zhao, Z. X. Wei, and B. H. Han, (2015) “Nitrogen-doped graphene aerogels as efficient supercapacitor electrodes and gas ad sorbents" ACSAppliedMaterialsandInterfaces7(3): 1431–1438. DOI: 10.1021/am5042065.
- [18] Y. Wang, M. Hu, D. Ai, H. Zhang, Z. H. Huang, R. Lv, and F. Kang, (2019) “Sulfur-doped reduced graphene oxide for enhanced sodium ion pseudocapacitance" Nano materials 9(5): DOI: 10.3390/nano9050752.
- [19] T. Jin, J. Chen, C. Wang, Y. Qian, and L. Lu, (2020) “Facile synthesis of fluorine-doped graphene aerogel with rich semi-ionic C–F bonds for high performance supercapacitor application" Journal of Materials Science 55(26): 12103–12113. DOI: 10.1007/s10853-020-04821-1.
- [20] P. Yan, L. Yan, S. Zhao, Z. Zuo, X. Wang, C. Wang, and M. Hou, (2019) “Fluorine-Doped Graphene/Nanosized Carbide-Derived Carbon Composites for High-Performance Supercapacitor" Nano 14(8): 1–11. DOI: 10.1142/S1793292019500991.
- [21] V. Thirumal, A. Pandurangan, R. Jayavel, and R. Ilangovan, (2016) “Synthesis and characterization of boron doped graphene nanosheets for supercapacitor applications" Synthetic Metals 220: 524–532. DOI: 10.1016/j.synthmet.2016.07.011.
- [22] W. Zhang, Z. Chen, X. Guo, K. Jin, Y. X. Wang, L. Li, Y. Zhang, Z. Wang, L. Sun, and T. Zhang, (2018) “N/S co-doped three-dimensional graphene hydrogel for high performance supercapacitor" Electrochimica Acta 278: 51–60. DOI: 10.1016/j.electacta.2018.05.018.
- [23] A. G. Kannan, J. Zhao, S. G. Jo, Y. S. Kang, and D. W. Kim, (2014) “Nitrogen and sulfur co-doped graphene counter electrodes with synergistically enhanced performance for dye-sensitized solar cells" Journal of Materials Chemistry A 2(31): 12232–12239. DOI: 10.1039/c4ta01927j.
- [24] Z. Lu, Y. Chen, Z. Liu, A. Li, D. Sun, and K. Zhuo, (2018) “Nitrogen and sulfur co-doped graphene aerogel for high performance supercapacitors" RSC Advances 8(34): 18966–18971. DOI: 10.1039/c8ra01715h.
- [25] Z. Ouyang, Y. Lei, Y. Chen, Z. Zhang, Z. Jiang, J. Hu, and Y. Lin, (2019) “Preparation and Specific Capacitance Properties of Sulfur, Nitrogen Co-Doped Graphene Quan tum Dots" Nanoscale Research Letters 14(1): DOI: 10.1186/s11671-019-3045-4.
- [26] X. Qiao, S. Liao, G. Wang, R. Zheng, H. Song, and X. Li, (2016) “Simultaneous doping of nitrogen and fluorine into reduced graphene oxide: A highly active metal-free electrocatalyst for oxygen reduction" Carbon 99: 272 279. DOI: https://doi.org/10.1016/j.carbon.2015.12.034.
- [27] Y. Liu, Q. Feng, Q. Xu, M. Li, N. Tang, and Y. Du, (2013) “Synthesis and photoluminescence of F and N co doped reduced graphene oxide" Carbon 61: 436–440. DOI: https://doi.org/10.1016/j.carbon.2013.05.027.
- [28] G. S. S. Mamaril, M. D. G. de Luna, K. Bindumadha van, D. C. Ong, J. A. I. Pimentel, and R. A. Doong, (2021) “Nitrogen and fluorine co-doped 3-dimensional reduced graphene oxide architectures as high-performance electrode material for capacitive deionization of copper ions" Separation and Purification Technology 272: 117559. DOI: 10.1016/j.seppur.2020.117559.
- [29] N. P. D. Ngidi, E. Muchuweni, and V. O. Nyamori, (2021) “Dual heteroatom-doped reduced graphene oxide and its application in dye-sensitized solar cells" Optical Materials 122: 111689. DOI: https://doi.org/10.1016/j.optmat.2021.111689.
- [30] X. Liu, Z. Lu, X. Huang, J. Bai, C. Li, C. Tu, and X. Chen, (2021) “Self-assembled S, N co-doped reduced graphene oxide/MXene aerogel for both symmetric liquid and all-solid-state supercapacitors" Journal of Power Sources 516: 230682. DOI: https://doi.org/10.1016/j.jpowsour.2021.230682.
- [31] S. Surya, A. Pandurangan, and R. Govindaraj, (2024) “Electrochemical investigation of phosphorous and boron heteroatoms incorporated reduced graphene oxide electrode material for supercapacitor applications" Journal of Energy Storage 86: 111319. DOI: https://doi.org/10.1016/j.est.2024.111319.
- [32] K. S. Rawat, C. Tewari, T. Arya, Y. N. Kim, P. Pant, S. Sati, S. Dhali, P. B. Negi, Y. C. Jung, and N. G. Sahoo, (2025) “Development of nitrogen and phosphorus dual-doped reduced graphene oxide from waste plastic for supercapacitor applications: Comparative electrochemical performance in different electrolytes" Next Energy 6: 100209. DOI: https://doi.org/10.1016/j.nxener.2024.100209.
- [33] P. B. Arthi G and L. BD, (2015) “A Simple Approach to Stepwise Synthesis of Graphene Oxide Nanomaterial" Journal of Nanomedicine &Nanotechnology06(01): 1–4. DOI: 10.4172/2157-7439.1000253.
- [34] N. I. Zaaba, K. L. Foo, U. Hashim, S. J. Tan, W. W. Liu, and C. H. Voon, (2017) “Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence" Procedia Engineering 184: 469–477. DOI: 10.1016/j.proeng.2017.04.118.
- [35] S. Thoufeeq, P. K. Rastogi, N. Sreekanth, M. M. R. I. Anantharaman, and T. N. Narayanan, (2018) “Nickel reduced graphene oxide composite foams for electrochemical oxidation processes: towards biomolecule sensing" MRS Communications 8(3): 695–702. DOI: DOI:10.1557/mrc.2018.123.
- [36] X. Lin, Y. Ni, and S. Kokot, (2012) “Voltammetric analysis with the use of a novel electro-polymerised graphene nafionfilm modified glassy carbon electrode: Simultane ous analysis of noxious nitroaniline isomers" Journal of Hazardous Materials 243: 232–241. DOI: https://doi.org/10.1016/j.jhazmat.2012.10.026.
- [37] Q. He, J. Liu, X. Liu, G. Li, D. Chen, P. Deng, and J. Liang, (2018) “Fabrication of Amine-Modified Magnetite Electrochemically Reduced Graphene Oxide Nanocomposite Modified Glassy Carbon Electrode for Sensitive Dopamine Determination" Nanomaterials 8(4): DOI: 10.3390/nano8040194.
- [38] D. Sánchez-Campos, V. Rodríguez-Lugo, F. C. Sánchez-Vargas, D. Mendoza-Anaya,E.S.Rodríguez, L. E. Alarcón, and M. I. Reyes-Valderrama, (2020) “Simple process and uncomplicated reduction of graphene oxide" Materials Chemistry and Physics 242: 122325. DOI: https://doi.org/10.1016/j.matchemphys.2019.122325.
- [39] A. Nugroho, F. Erviansyah, D. Floresyona, S. Mahalingam, A. Manap, N. Afandi, K. S. Lau, and C. H. Chia, (2022) “Synthesis and Characterization NS Reduced Graphene Oxide Hydrogel and Its Electrochemical Properties" Letters on Materials 12(2): 169–174. DOI: 10.22226/2410-3535-2022-2-169-174.
- [40] K. Kakaei and A. Balavandi, (2017) “Hierarchically porous fluorine-doped graphene nanosheets as efficient metal-free electrocatalyst for oxygen reduction in gas diffusion electrode" Journal of Colloid and Interface Science 490: 819–824. DOI: https://doi.org/10.1016/j.jcis.2016.12.011.
- [41] A. Alkhouzaam, H. Abdelrazeq, M. Khraisheh, F. AlMomani, B. H. Hameed, M. K. Hassan, M. A. Al Ghouti, and R. Selvaraj. Spectral and Structural Proper ties of High-Quality Reduced Graphene Oxide Produced via a Simple Approach Using Tetraethylenepentamine. 2022. DOI: 10.3390/nano12081240.
- [42] U. Chasanah, W. Trisunaryanti, T. Triyono, H. Okta viano, I. Santoso, and D. Fatmawati, (2022) “Study of green reductant effects of highly reduced graphene ox ide production and their characteristics" Communications in Science and Technology 7: 103–111. DOI: 10.21924/cst.7.2.2022.906.
- [43] R. Paul, F. Du, L. Dai, Y. Ding, Z. L. Wang, F. Wei, and A. Roy, (2019) “3D Heteroatom-Doped Carbon Nanomaterials as Multifunctional Metal-Free Catalysts for Inte grated Energy Devices" Advanced Materials 31(13): 1805598. DOI: https://doi.org/10.1002/adma.201805598.
- [44] V. Wardani, L. Rohmawati, W. Setyarsih, D. Alfarisi, and A. Subhan, (2020) “Analysis of Charging/Discharging Supercapacitor Active Carbon/rGO Based on Natural Materials" Journal of Physics: Conference Series 1491(1): 12044. DOI: 10.1088/1742-6596/1491/1/012044.
- [45] Q. Liu, Q. Shi, H. Wang, Q. Zhang, and Y. Li, (2015) “Laser irradiated self-supporting and flexible 3-dimentional graphene-based film electrode with promising electrochemical properties" RSC Advances 5(58): 47074–47079. DOI: 10.1039/C5RA08431H.