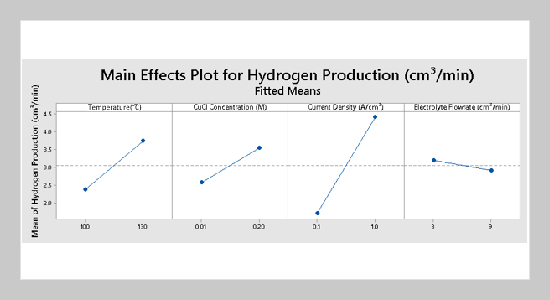
Mohd Fadhzir Ahmad Kamaroddin1,2,3This email address is being protected from spambots. You need JavaScript enabled to view it., Nordin Sabli1,4This email address is being protected from spambots. You need JavaScript enabled to view it., Tuan Amran Tuan Abdullah2,3, Luqman Chuah Abdullah1, Shamsul Izhar1, Nurul Sahida Hassan2, and Aishah Abdul Jalil2,3
1Department of Chemical and Environmental Engineering, Faculty of Engineering, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
2Centre of Hydrogen Energy, Institute of Future Energy, Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia
3Faculty of Chemical and Energy Engineering, Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia
4Institute of Nanoscience and Nanotechnology (IONS), Universiti Putra Malaysia, 43400, UPM Serdang, Selangor, Malaysia
- [1] I. K. Muritala, D. Guban, M. Roeb, and C. Sattler, (2020) “High Temperature Production of Hydrogen: Assessment of Non-Renewable Resources Technologies and Emerging Trends" International Journal of Hydrogen Energy 45(49): 26022–26035. DOI: 10.1016/j.ijhydene.2020.06.123.
- [2] S. Kim, R. Schatz, S. Khurana, M. Fedkin, C. Wang, andS.Lvov,(2019)“AdvancedCuClElectrolyzerforHy drogen Production via the Cu–Cl Thermochemical Cycle" ECS Transactions 35(32): 257. DOI: 10.1149/05001.0257ecst.
- [3] D. M. Hall and S. N. Lvov, (2016) “Modeling a CuCl (aq)/HCl (aq) Electrolyzer Using Thermodynamics and Electrochemical Kinetics" Electrochimica Acta 190: 1167–1174. DOI: 10.1016/j.electacta.2015.12.123.
- [4] O. A. Jianu, Z. Wang, M. A. Rosen, and G. F. Naterer, (2013) “Shadow Imaging of Particle Dynamics and Dissolution Rates in Aqueous Solutions for Hydrogen Production" Experimental Thermal and Fluid Science 51: 297–301. DOI: 10.1016/j.expthermflusci.2013.09.045.
- [5] A. Farsi, C. Zamfirescu, I. Dincer, and G. F. Naterer, (2019) “Thermodynamic Assessment of a Lab-Scale Experimental Copper-Chlorine Cycle for Sustainable Hydrogen Production" International Journal of Hydrogen Energy 44(33): 17595–17610. DOI: 10.1016/j.ijhydene.2019.04.123.
- [6] A.Farsi, " Kayhan, C. Zamfirescu, I. Dincer, and G. F. Naterer, (2019) “Kinetic and Hydrodynamic Analyses of Chemically Reacting Gas-Particle Flow in Cupric Chlo ride Hydrolysis for the Cu–Cl Cycle" International Journal of Hydrogen Energy 44(49): 26783–26793. DOI: 10.1016/j.ijhydene.2019.05.678.
- [7] D. Bessarabov, H. Wang, H. Li, and N. Zhao, eds. PEM Electrolysis for Hydrogen Production: Principles and Applications. Boca Raton, FL: CRC Press, 2016. DOI: 10.1201/b20378.
- [8] J. Ran, L. Wu, Y. He, Z. Yang, Y. Wang, C. Jiang, and T. Xu, (2017) “Ion Exchange Membranes: New Developments and Applications" Journal of Membrane Science 522: 267–291. DOI: 10.1016/j.memsci.2017.02.011.
- [9] Q. Zhang, H. Liu, X. Li, R. Xu, J. Zhong, R. Chen, and X. Gu, (2016) “Synthesis and Characterization of Polybenzimidazole/α-Zirconium Phosphate Composites as Proton Exchange Membrane" Polymer Engineering & Science 56(6): 622–628. DOI: 10.1002/pen.24367.
- [10] Z. Zhou, O. Zholobko, X. F. Wu, T. Aulich, J. Thakare, and J. Hurley, (2020) “Polybenzimidazole-Based Polymer Electrolyte Membranes for High-Temperature Fuel Cells: Current Status and Prospects" Energies 14(1): 135. DOI: 10.3390/en14010135.
- [11] D. Aili, M. K. Hansen, C. Pan, Q. Li, E. Christensen, J. O. Jensen, and N. J. Bjerrum, (2011) “Phosphoric Acid Doped Membranes Based on Nafion®, PBI and Their Blends–Membrane Preparation, Characterization and Steam Electrolysis Testing" International Journal of HydrogenEnergy36(12):6985–6993. DOI: 10.1016/j.ijhydene.2011.03.058.
- [12] Z. Mossayebi, T. Saririchi, S. Rowshanzamir, and M. J. Parnian, (2016) “Investigation and Optimization of Physicochemical Properties of Sulfated Zirconia/Sulfonated Poly (Ether Ether Ketone) Nanocomposite Membranes for Medium Temperature Proton Exchange Membrane Fuel Cells" International Journal of Hydrogen Energy 41(28): 12293–12306. DOI: 10.1016/j.ijhydene.2016.05.183.
- [13] A. Villagra and P. Millet, (2019) “An Analysis of PEM Water Electrolysis Cells Operating at Elevated Current Densities" International Journal of Hydrogen Energy 44(20): 9708–9717. DOI: 10.1016/j.ijhydene.2019. 02.120.
- [14] F. Gashoul, M. J. Parnian, and S. Rowshanzamir, (2017) “A New Study on Improving the Physicochemical and Electrochemical Properties of SPEEK Nanocomposite Membranes for Medium Temperature Proton Exchange Membrane Fuel Cells Using Different Loading of Zirconium Oxide Nanoparticles" International Journal of Hydrogen Energy 42(1): 590–602. DOI: 10.1016/j. ijhydene.2016.09.124.
- [15] A. Iulianelli and A. Basile, (2012) “Sulfonated PEEK Based Polymers in PEMFC and DMFC Applications: A Review" International Journal of Hydrogen Energy 37(20): 15241–15255. DOI: 10.1016/j.ijhydene.2012.06.031.
- [16] N. Shaari and S. K. Kamarudin, (2019) “Recent Advances in Additive-Enhanced Polymer Electrolyte Mem brane Properties in Fuel Cell Applications: An Overview" International Journal of Energy Research 43(7): 2756–2794. DOI: 10.1002/er.4405.
- [17] N.AbdoandE.B.Easton, (2016) “Nafion/Polyaniline Composite Membranes for Hydrogen Production in the Cu–Cl Thermochemical Cycle" International Journal of HydrogenEnergy41(19):7892–7903. DOI: 10.1016/j.ijhydene.2015.11.180.
- [18] S. Giddey, S. P. S. Badwal, and H. Ju. “Polymer Electrolyte Membrane Technologies Integrated with Renewable Energy for Hydrogen Production”. In: Current Trends and Future Developments on (Bio-) Mem branes. Elsevier, 2019, 235–259. DOI: 10.1016/B978-0-12-814604-5.00010-6.
- [19] N. Sathaiyan, V. Nandakumar, G. Sozhan, J. G. Packiaraj, E. T. Devakumar, D. Parvatalu, and B. N. Prabhu, (2015) “Hydrogen Generation Through Cuprous Chloride-Hydrochloric Acid Electrolysis" International Journal of Energy Power Engineering 4: 15–22.
- [20] M. F. A. Kamaroddin, N. Sabli, P. M. Nia, T. A. T. Abdullah, L. C. Abdullah, S. Izhar, and A. Ahmad, (2020) “Phosphoric Acid Doped Composite Proton Ex change Membrane for Hydrogen Production in Medium Temperature Copper Chloride Electrolysis" International Journal of Hydrogen Energy 45(42): 22209–22222. DOI: 10.1016/j.ijhydene.2020.06.050.
- [21] J. Escorihuela, J. Olvera-Mancilla, L. Alexandrova, L. F. Del Castillo, and V. Compañ, (2020) “Recent Progress in the Development of Composite Membranes Based on Polybenzimidazole for High Temperature Pro ton Exchange Membrane (PEM) Fuel Cell Applications" Polymers 12(9): 1861. DOI: 10.3390/polym12091861.
- [22] Y. Xiao, X. Shen, R. Sun, S. Wang, J. Xiang, L. Zhang, and N. Tang, (2022) “Polybenzimidazole Mem brane Crosslinked with Quaternized Polyaniline as High Temperature Proton Exchange Membrane: Enhanced Pro ton Conductivity and Stability" Journal of Membrane Science 660: 120795. DOI: 10.1016/j.memsci.2022. 120795.
- [23] H. Li, L. Stolberg, A. Vega, W. Zhang, S. Reinwald, D. Ryland, and S. Suppiah, (2020) “Canadian Advances in the Copper–Chlorine Thermochemical Cycle for Clean Hydrogen Production: A Focus on Electrolysis" International Journal of Hydrogen Energy 45(58): 33037 33046. DOI: 10.1016/j.ijhydene.2020.05.232.
- [24] M. F. A. Kamaroddin, N. Sabli, T. A. T. Abdullah, S. I. Siajam, L. C. Abdullah, A. A. Jalil, and A. Ah mad,(2021) “Membrane-Based Electrolysis for Hydrogen Production: A Review" Membranes 11(11): 810. DOI: 10.3390/membranes11110810.
- [25] N. I. Madondo, S. Rathilal, and B. F. Bakare, (2022) “Utilization of Response Surface Methodology in Optimization and Modelling of a Microbial Electrolysis Cell for Wastewater Treatment Using Box–Behnken De sign Method" Catalysts 12(9): 1052. DOI: 10.3390/ catal12091052.
- [26] E. H. Gürkan, Y. Tibet, and S. Çoruh, (2021) “Application of Full Factorial Design Method for Optimization of Heavy Metal Release from Lead Smelting Slag" Sustain ability 13(9): 4890. DOI: 10.3390/su13094890.
- [27] M. F. A. Kamaroddin, N. Sabli, P. M. Nia, T. A. T. Abdullah, L. C. Abdullah, S. Izhar, and A. Ahmad, (2020) “Phosphoric Acid Doped Composite Proton Ex change Membrane for Hydrogen Production in Medium Temperature Copper Chloride Electrolysis" International Journal of Hydrogen Energy 45(42): 22209–22222. DOI: 10.1016/j.ijhydene.2020.06.050.
- [28] R. Soltani, I. Dincer, and M. A. Rosen, (2019) “Kinetic and Electrochemical Analyses of a CuCl/HCl Electrolyzer" International Journal of Energy Research 43(13): 6890–6906. DOI: 10.1002/er.4395.
- [29] S. Toghyani, E. Afshari, E. Baniasadi, S. A. Atyabi, and G. F. Naterer, (2018) “Thermal and Electrochemical Performance Assessment of a High-Temperature PEM Electrolyzer" Energy 152: 237–246. DOI: 10.1016/j.energy.2018.03.140.
- [30] S. Toghyani, E. Afshari, and E. Baniasadi, (2018) “Metal Foams as Flow Distributors in Comparison with Serpentine and Parallel Flow Fields in Proton Exchange Membrane Electrolyzer Cells" Electrochimica Acta 290: 506–519. DOI: 10.1016/j.electacta.2018.09.056.
- [31] F. Scheepers, M. Stähler, A. Stähler, E. Rauls, M. Müller, M. Carmo, and W. Lehnert, (2021) “Temper ature Optimization for Improving Polymer Electrolyte Membrane-Water Electrolysis System Efficiency" Ap plied Energy 283: 116270. DOI: 10.1016/j.apenergy. 2020.116270.