REFERENCES
- [1] Venkataraman, D.; Lee, S.; Zhang, J.; Moore, J. S. Nature 1994, 371, 591.
- [2] (a) Chui, S. S.-Y.; Lo, S. M.-F.; Charmant, J. P. H.; Orpen, A. G.; Williams, I. D. Sci. 1999, 283, 1148; (b) Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O. M. Nature 1999, 402, 276; (c) Seo, J. S.; Whang, D.; Lee, H.; Jun, S. I.; Oh, J.; Jeon, Y. J.; Kim, K. Nature 2000, 404, 982.
- [3] Orr, W. G.; Barbour, L. J.; Atwood, J. L. Sci. 1999, 285, 1049.
- [4] (a) Diskin-Posner, Y.; Dahal, S.; Goldberg, I. Angew. Chem. Int. Ed. 2000, 39, 1288, and reference therein; (b) Abrahams, B. F.; Hoskins, B. F.; Michail, D. M.; Robson, R. Nature 1994, 369, 727; (c) Belanger, S.; Hupp, J. T. Angew. Chem. 1999, 111, 2360; Angew. Chem. Int. Ed. 1999, 38, 2222; (d) Drain, C. M.; Nifiatis, F.; Vasenko, A.; Batteas, J. D. Angew. Chem. 1998, 110, 2478; Angew. Chem. Int. Ed. 1998, 37, 2344.
- [5] Nakano, A.; Osuka, A.; Yamazaki, I.; Yamazaki, T.; Nishimura, Y. Angew. Chem. 1998, 110, 3172; Angew. Chem. Int. Ed. 1998, 37, 3023.
- [6] (a) Lin, K.-J.; Lii, K. H. Angew. Chem. 1997, 109, 2166; Angew. Chem. Int. Ed. 1997, 36, 2076; (b) Lin, K.-J. Angew. Chem. 1999, 111, 2894; Angew. Chem. Int. Ed. 1999, 38, 2730.
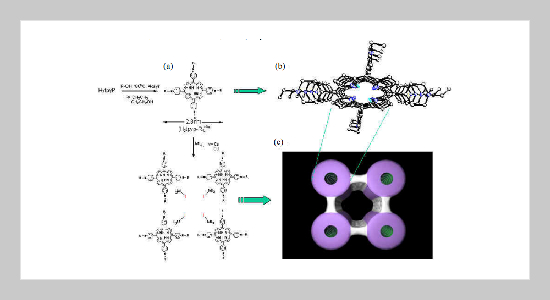
- [7] Synthesis of SMTP-2(1): A mixture of H2tpyp (0.0732 g), CdI2 (0.0619 g), I2 (0.3 g), CH3COOH (4 mL) and Ethanol (4 mL) was sealed in a 23-mL Telflon-lined stainless autoclave, heating to 180 o C for 48 h, and cooled to 70 o C at 6 Kh-1. Needle-shaped purple crystals were filtered of and wash with ethanol. The yield of crystalline material was 87 % (0.1847 g) based on H2tpyp, and the synthesis was highly reproducible. Elemental analysis (%): calcad: C 29.00, N 5.41, O 3.09, H 2.63; found: C 28.21, N 5.42, O 3.40, H 2.92.
- [8] X-ray data for SMTP-2(1) : tetragonal, space group P42/n, a = b = 29.078(3) Å, c = 8.134(1) Å, V = 6872(1) Å3 , R1 = 0.065, wR2(F2 ) = 0.173, GOF = 0.943. The pore solvent molecules could not be completely located in the structure analysis. However, element analysis and results of the thermogravimetric analysis are in agreement in indicating four acetic acid molecules and eight water molecules per unit cell in these channels.. For SMTP-2(2), most needle-shaped crystals adhere small individual crystals and thus their diffraction qualities are poor. However, its tetragonal unit cell parameters, a = 28.598(2), b = 28.598(2), and c = 8.152(1) Å, was determined by a least-square fit of selected 344 reflections with 6 < 2θ < 40o on a BRUKER smart-CCD diffractometer. Furthermore, the homogeneity of two bulk products (SMTP-2(1) and SMTP-2(2)) was confirmed by comparison of the observed and calculated powder patterns derived from single crystal structure analysis as well as by comparison of the IR and NMR-spectra.
- [9] After immersion of 5 mg of SMTP-2 in a solution containing 10 ppm lead ions for 3 h, only 10.27 % lead was sorbed by employing anodic stripping voltammetry methods.
- [10] (a) Hunter, C. A.; Hyde, R. K. Angew. Chem. 1996, 108, 2064; Angew. Chem. Int. Ed. 1996, 35, 1936; (b) Ashton, P. R.; Shibata, K.; Shipway, A. N.; Stoddart, J. F. Angew. Chem. 1997, 109, 2902; Angew. Chem. Int. Ed. 1997, 36, 2781; (c) Ruhlmann, L.; Schulz, A.; Giraudeau, A.; Messerschmidt, C.; Fuhrhop, J. -H. J. Am. Chem. Soc. 1999, 121, 6664.
- [11] Crystals of SMTP-2 bonded into an electrode form is immersed in a polar solvent in which magnesium sulfate is dissolved. A sheet of magnesium metal serves as the anode. On shorting the two electrodes, magnesium ions intercalate SMTP-2, the charge-compensating electrons passing through the external circuit (50 V, 0.1 mA).
- [12] Sharma, C. V. K.; Broker, G. A.; Huddleston, J. G.; Baldwin, J. W.; Metzger, R. M.; Rogers, R. D. J. Am. Chem. Soc. 1999, 121, 1137.