Trong-Ming Don This email address is being protected from spambots. You need JavaScript enabled to view it.1 , Chung-Yang Chuang2 and Wen-Yen Chiu2 1Department of Chemical Engineering Tamkang University, Tamsui, Taiwan 251, R.O.C.
2Department of Chemical Engineering National Taiwan University, Taipei, Taiwan 106, R.O.C.
Received:
October 16, 2002
Accepted:
November 11, 2002
Publication Date:
December 1, 2002
Download Citation:
||https://doi.org/10.6180/jase.2002.5.4.06
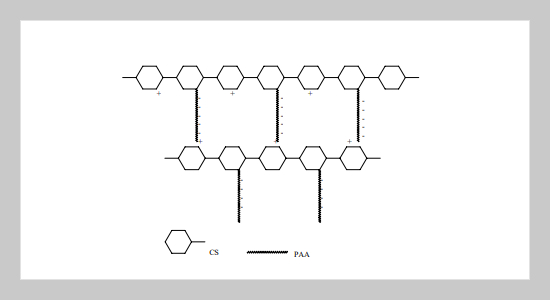
Graft copolymers of Chitosan and poly(acrylic acid) were synthesized with a redox initiator, cerium ammonium nitrate. After 2 h of reaction at 70 o C, a gel product was obtained. The swelling ratio of the copolymer in water depends on the pH value and has a maximum in the buffer solution at pH 7. This is because the morphological structure of the copolymers in the water changes with the pH values. In addition, the swelling ratio of the copolymer increases with the feeding amount of acrylic acid monomer. The degradation behavior of the copolymers was observed both in a lysozyme solution and an active slurry solution. The degradation rate was higher in the lysozyme solution and depended on the composition of the copolymers.ABSTRACT
Keywords:
Chitosan, Poly(acrylic acid), Graft Copolymer, Degradation, Lysozyme, Slurry
REFERENCES