Chia-Chun Chen This email address is being protected from spambots. You need JavaScript enabled to view it.1 and Chi-Hui Liang2 1Departments of Chemistry National Taiwan Normal University Taipei, Taiwan 116, R.O.C.
2Center for Condensed Matter Sciences National Taiwan University Taipei, Taiwan 106, R.O.C.
Received:
October 8, 2002
Accepted:
November 4, 2002
Publication Date:
December 1, 2002
Download Citation:
||https://doi.org/10.6180/jase.2002.5.4.04
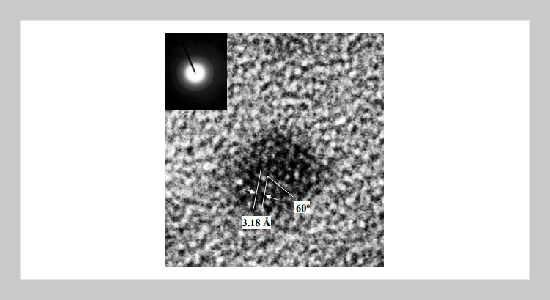
A solution phase synthetic method of colloidal GaN nanocrystals at the low temperature has been reported. The syntheses involve the reaction of gallium chloride and lithium nitride in aromatic type solvents mixed surfactants, trioctylphosphine (TOPO) at the temp- erature below 300 o C. The resulting nanocrystals were crystalline and generally spherical with the surfaces passivated by TOPO giving the individual nanocrystals solubility in common organic solvents. The average crystal sizes varied from 50 Å to 200 Å in different reaction conditions. We have confirmed the hexagonal wurzite structure of the GaN naocrystals by transmission electron miwcroscope (TEM), x-ray powder diffraction. The samples were examined the optical emission by an emission spectrometer. The results indicated that the nanocrystals emit photoluminescence at 3.31 eV.ABSTRACT
Keywords:
Nanocrystals, Gallium Nitride, GaN Nanocrystals, Zinc Blende
REFERENCES