Hung-Jen Huang1 and Jem-Mau Lo 1 1Department of Nuclear Science National Tsing Hua University Hsinchu, Taiwan 300, R.O.C
Received:
January 7, 2003
Accepted:
February 7, 2003
Publication Date:
March 1, 2003
Download Citation:
||https://doi.org/10.6180/jase.2003.6.1.03
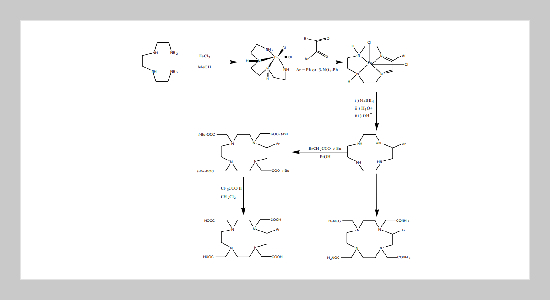
C-aryl derivatives of DOTA (N,N’,N”,N”’-tetrakis(carboxymethyl)- 1,4,7,10-tetraazacyclododecane) including phenyl-DOTA and 3-NO2- Ph-DOTA were synthesized by a template reductive reaction. 67GaCl3 or 68GaCl3 was taken and transformed into 67,68Ga-acetylacetone ( 67,68Ga(acac)3) before labeling to the DOTA derivatives. The 67Ga or 68Ga labeled complexes were generated by ligand-ligand exchange of acetylacetonate with the DOTA derivatives. The labeling efficiency of PhDOTA with 67,68Ga could be higher than 95% under the reaction being facilitated via sonication, at ambient temperature for 20 min. By a similar reaction, the labeling efficiency of 3-NO2-Ph-DOTA with 67,68Ga was found to be lower (~ 40%). The stability of 67Ga-Ph-DOTA was high, maintaining at ≥ 98% yield even at 24 h post-preparation. The 68Ga labeled Ph-DOTA complex was radiochemically characterized to be anionic and hydrophilic. It is anticipated from the result that 68Ga in the form of Ga(III) may be six coordinated with Ph-DOTA by 4N from the ring and 2O from two of four pendent acetic groups, leaving other two acetic groups uncoordinated.ABSTRACT
Keywords:
67Ga, 68Ga, DOTA, 67,68Ga-DOTA Derivatives, RadioChemical Characterization
REFERENCES