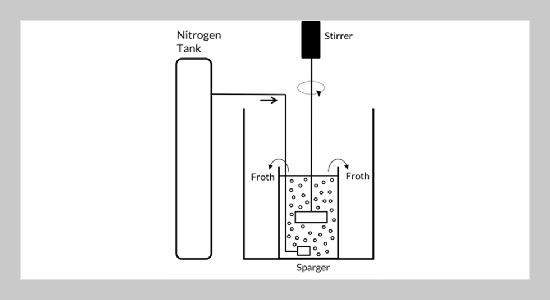
Kevin Cleary Wanta1, Alexander William Prijadi1, Himawan Tri Bayu Murti Petrus2, Arifudin Idrus3, Iga Trisnawati4, Agus Saptoro5, and Ratna Frida Susanti1This email address is being protected from spambots. You need JavaScript enabled to view it.
1Department of Chemical Engineering, Faculty of Industrial Technology, Parahyangan Catholic University, Jl. Ciumbuleuit 94, Bandung, 40141, Indonesia
2Department of Chemical Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jl. Grafika No. 2, Kampus UGM, Yogyakarta, 55281, Indonesia
3Department of Geological Engineering, Faculty of Engineering, Universitas Gadjah Mada, Jl. Grafika No. 2, Kampus UGM, Yogyakarta, 55281, Indonesia
4Polytechnic Institute of Nuclear Technology, National Research and Innovation Agency, Jl. Babarsari Kotak POB 6101/YKBB, Yogyakarta, Indonesia 55281
5Department of Chemical Engineering, Curtin University Malaysia, Miri, Sarawak, Malaysia
- [1] J. P. Rafferty et al. Minerals. Britannica Educational Publishing, 2011.
- [2] C. Ogwata and M. Onwughalu, (2019) “Occurrence of galena and its potentials for economic and green energy revolution in Nigeria" IRE J 3: 139–142.
- [3] L. George, N. J. Cook, C. Cristiana L, and B. P. Wade, (2015) “Trace and minor elements in galena: A recon�naissance LA-ICP-MS study" American Mineralogist 100(2-3): 548–569. DOI: 10.2138/am-2015-4862.
- [4] I. A. Nnanwube and O. D. Onukwuli, (2020) “Mod�eling and optimization of galena dissolution in a binary solution of nitric acid and ferric chloride using artificial neural network coupled with genetic algorithm and re�sponse surface methodology" South African Journal of Chemical Engineering 32: 68–77. DOI: 10.1016/j.sajce.2020.03.001.
- [5] F. Nikkhou, F. Xia, M. Knorsch, and A. P. Dedi�tius, (2020) “Mechanisms of surface passivation during galena leaching by hydrogen peroxide in acetate and citrate solutions at 25–50 C" ACS Sustainable Chemistry & Engineering 8(38): 14407–14416. DOI: 10.1021/acssuschemeng.0c04272.
- [6] K. L. Pruseth, N. Jehan, P. Sahu, and B. Mishra, (2014) “The possibility of a ZnS-bearing sulfide melt at 600 C: Evidence from the Rajpura–Dariba deposit, India, sup�ported by laboratory melting experiment" Ore Geology Reviews 60: 50–59. DOI: 10.1016/j.oregeorev.2013.12.012.
- [7] A. A. Baba and F. A. Adekola, (2012) “A study of dis�solution kinetics of a Nigerian galena ore in hydrochloric acid" Journal of Saudi Chemical Society 16(4): 377–386. DOI: 10.1016/j.jscs.2011.02.005.
- [8] B. S. Al-Saqarat, A. Al-Mobydeen, A. N. Al-Masri, M. Esaifan, I. Hamadneh, I. S. Moosa, and E. Al�Shamaileh, (2023) “Facile Production Method of PbS Nanoparticles via Mechanical Milling of Galena Ore" Mi�cromachines 14(3): 564. DOI: 10.3390/mi14030564.
- [9] I. A. Nnanwube, O. D. Onukwuli, et al., (2018) “Hy�drometallurgical processing of a Nigerian galena ore in ni�tric acid: Characterization and dissolution kinetics" Jour�nal of Minerals and Materials Characterization and Engineering 6(03): 271. DOI: 10.4236/jmmce.2018.63020.
- [10] S. W. Merkel, (2021) “Evidence for the widespread use of dry silver ore in the Early Islamic period and its impli�cations for the history of silver metallurgy" Journal of Archaeological Science 135: 105478. DOI: 10.1016/j.jas.2021.105478.
- [11] M. Raza, M. Bhatti, S. Nasir, F. Bashir, Z. Mahmood, K. Kazmi, and I. Hafeez, (2019) “Study on low-grade galena-barite ore beneficiation in Khuzdar, Balochistan, Pakistan" Mining of mineral deposits: DOI: 10.33271/mining13.01.001.
- [12] A. Idrus, L. D. Setijadji, F. Tamba, and F. Anggara, (2011) “Geology and characteristics of Pb-Zn-cu-ag skarn deposit at Ruwai, Lamandau regency, Central Kaliman�tan" Journal of Applied Geology 3(1): DOI: 10.17014/ijog.v6i4.126.
- [13] F. Feng, W. Liu, S. Liu, and S. Chen, (2022) “Mineral�ogy and innovative flash flotation separation of Cu-Pb-Zn polymetallic ore in weak acidic pulp" Minerals 12(8): 1041. DOI: 10.3390/min12081041.
- [14] G. Tiu, Y. Ghorbani, N. Jansson, and C. Wanhainen, (2021) “Tracking silver in the Lappberget Zn-Pb-Ag-(Cu�Au) deposit, Garpenberg mine, Sweden: Towards a ge�ometallurgical approach" Minerals Engineering 167: 106889. DOI: 10.1016/j.mineng.2021.106889.
- [15] Y.-h. Qin, G. Peng, Y. Shuai, N.-y. Zhang, and L.-r. Han, (2021) “A novel technology of high-voltage pulse discharge for comminution of galena ore" Transactions of Nonferrous Metals Society of China 31(8): 2479–2492. DOI: 10.1016/S1003-6326(21)65668-6.
- [16] D. Zhai, J. Liu, N. J. Cook, X. Wang, Y. Yang, A. Zhang, and Y. Jiao, (2019) “Mineralogical, textural, sulfur and lead isotope constraints on the origin of Ag�Pb-Zn mineralization at Bianjiadayuan, Inner Mongo�lia, NE China" Mineralium Deposita 54: 47–66. DOI: 10.1007/s00126-018-0804-6.
- [17] H. Ba¸stürkcü, Ü. Yenial, O. Kökkılıç, A. E. Yüce, and E. B. Erdo˘gan. “Beneficiation of copper, lead and zinc concentrates from complex ore by using environmen�tally friend reagents”. In: The XIII international mineral processing symposium. Bodrum, Turkey. 2012, 349–355.
- [18] L. Zhang, J. Gao, S. A. Khoso, L. Wang, Y. Liu, P. Ge, M. Tian, and W. Sun, (2021) “A reagent scheme for galena/sphalerite flotation separation: Insights from first�principles calculations" Minerals Engineering 167: 106885. DOI: 10.1016/j.mineng.2021.106885.
- [19] Q. Xuemin, Y. Hongying, C. Guobao, Z. Shuiping, C. Chuangkai, and L. Bibo, (2020) “Inhibited mecha�nism of carboxymethyl cellulose as a galena depressant in chalcopyrite and galena separation flotation" Minerals Engineering 150: 106273. DOI: 10.1016/j.mineng.2020.106273.
- [20] X. Long, Y. Chen, J. Chen, Z. Xu, Q. Liu, and Z. Du, (2016) “The effect of water molecules on the thiol collec�tor interaction on the galena (PbS) and sphalerite (ZnS) surfaces: A DFT study" Applied Surface Science 389: 103–111. DOI: 10.1016/j.apsusc.2016.07.084.
- [21] H. Xie, Y. Liu, B. Rao, L. Gao, L. Chen, X. Tian, et al., (2021) “Selective passivation behavior of galena surface by sulfuric acid and a novel flotation separation method for copper-lead sulfide ore without collector and inhibitor" Separation and Purification Technology 267: 118621. DOI: 10.1016/j.seppur.2021.118621.
- [22] S. Li, M. P. Schwarz, Y. Feng, P. Witt, and C. Sun, (2019) “A CFD study of particle–bubble collision effi�ciency in froth flotation" Minerals Engineering 141: 105855. DOI: 10.1016/j.mineng.2019.105855.
- [23] F. Pita and A. Castilho, (2017) “Separation of plastics by froth flotation. The role of size, shape and density of the particles" Waste Management 60: 91–99. DOI: 10. 1016/j.wasman.2016.07.041.
- [24] B. Shean and J. Cilliers, (2011) “A review of froth flota�tion control" International Journal of Mineral Pro�cessing 100(3-4): 57–71. DOI: 10.1016/j.minpro.2011.05.002.
- [25] G. Wang, A. V. Nguyen, S. Mitra, J. Joshi, G. J. Jame�son, and G. M. Evans, (2016) “A review of the mecha�nisms and models of bubble-particle detachment in froth flotation" Separation and Purification Technology 170: 155–172. DOI: 10.1016/j.seppur.2016.06.041.
- [26] S. Özün and G. Ergen, (2019) “Determination of op�timum parameters for flotation of galena: effect of chain length and chain structure of xanthates on flotation re�covery" ACS omega 4(1): 1516–1524. DOI: 10.1021/acsomega.8b02841.
- [27] P. Quintanilla, S. J. Neethling, and P. R. Brito-Parada, (2021) “Modelling for froth flotation control: A review" Minerals Engineering 162: 106718. DOI: 10.1016/j.mineng.2020.106718.
- [28] S. Farrokhpay, L. Filippov, and D. Fornasiero, (2021) “Flotation of fine particles: A review" Mineral Processing and Extractive Metallurgy Review 42(7): 473–483. DOI: 10.1080/08827508.2020.1793140.
- [29] D. Mesa and P. R. Brito-Parada, (2019) “Scale-up in froth flotation: A state-of-the-art review" Separation and Purification Technology 210: 950–962. DOI: 10.1016/j.seppur.2018.08.076.
- [30] A. W. Prijadi, K. C. Wanta, H. T. B. M. Petrus, A. Idrus, and R. F. Susanti, (2024) “Ruwai Galena Ore Purification Through Column Flotation Process" Trans�actions of the Indian Institute of Metals: 1–9. DOI: 10.1007/s12666-024-03289-w.
- [31] Y. Yu, L. Ma, M. Cao, and Q. Liu, (2017) “Slime coat�ings in froth flotation: A review" Minerals Engineering 114: 26–36. DOI: 10.1016/j.mineng.2017.09.002.
- [32] A. S. Afolabi, A. S. Abdulkareem, and E. Muzenda, (2013) “Effect of flotation parameters on recovery of South Africa nickel sulphide ore" Applied Mechanics and Materials 260: 961–968. DOI: 10.4028/www.scientific.net/AMM.260-261.961.
- [33] M. Zhang and Y. Peng, (2015) “Effect of clay minerals on pulp rheology and the flotation of copper and gold minerals" Minerals Engineering 70: 8–13. DOI: 10.1016/j.mineng.2014.08.014.
- [34] E. Forbes, K. Davey, and L. Smith, (2014) “Decoupling rehology and slime coatings effect on the natural flotability of chalcopyrite in a clay-rich flotation pulp" Minerals Engineering 56: 136–144. DOI: 10.1016/j.mineng.2013.11.012.
- [35] E. Jorjani, H. Barkhordari, M. T. Khorami, and A. Fazeli, (2011) “Effects of aluminosilicate minerals on copper–molybdenum flotation from Sarcheshmeh por�phyry ores" Minerals Engineering 24(8): 754–759. DOI: 10.1016/j.mineng.2011.01.005.
- [36] C. Weiyong, C. Jianhua, L. Yuqiong, C. Ye, and Z. Cuihua, (2020) “Interactions of xanthate molecule with different mineral surfaces: A comparative study of Fe, Pb and Zn sulfide and oxide minerals with coordination chemistry" Minerals Engineering 159: 106565. DOI: 10.1016/j.mineng.2020.106565.
- [37] A. Foroutan, M. Abbas Zadeh Haji Abadi, Y. Kianinia, and M. Ghadiri, (2021) “Critical importance of pH and collector type on the flotation of sphalerite and galena from a low-grade lead–zinc ore" Scientific reports 11(1): 3103. DOI: 10.1038/s41598-021-91063-z.
- [38] S. Mondal, A. Acharjee, U. Mandal, and B. Saha, (2021) “Froth flotation process and its application" Viet�nam Journal of Chemistry 59(4): 417–425. DOI: 10.1002/vjch.202100010.
- [39] Z. Dong, T. Jiang, B. Xu, Q. Li, H. Zhong, and Y. Yang, (2021) “Selective flotation of galena using a novel collector S-benzyl-N-ethoxycarbonyl thiocarbamate: An experimen�tal and theoretical investigation" Journal of Molecular Liquids 330: 115643. DOI: 10.1016/j.molliq.2021.115643.
- [40] M. R. Mahmoud, N. K. Lazaridis, and K. A. Matis, (2015) “Study of flotation conditions for cadmium (II) removal from aqueous solutions" Process Safety and Environmental Protection 94: 203–211. DOI: 10.1016/j.psep.2014.06.012.
- [41] Z. J. Ang, G. Bournival, and S. Ata, (2013) “Influence of frothers on the detachment of galena particles from bub�bles" International Journal of Mineral Processing 121: 59–64. DOI: 10.1016/j.minpro.2013.02.003.
- [42] F. Cappuccitti and J. Nesset. “Frother and collector effects on flotation cell hydrodynamics and their im�plication on circuit performance”. In: Proceedings 48th Conference of Metallurgists of CIM. Sudbury. 2009, 169–180.
- [43] Y.-S. Cho and J. Laskowski, (2002) “Effect of flotation frothers on bubble size and foam stability" International Journal of Mineral Processing 64(2-3): 69–80. DOI: 10.1016/S0301-7516(01)00064-3.
- [44] P. Pawliszak, B. H. Bradshaw-Hajek, W. Skinner, D. A. Beattie, and M. Krasowska, (2024) “Frothers in flota�tion: A review of performance and function in the context of chemical classification" Minerals Engineering 207: 108567. DOI: 10.1016/j.mineng.2023.108567.
- [45] Y. Hu, M. Wu, R. Liu, and W. Sun, (2020) “A review on the electrochemistry of galena flotation" Minerals Engineering 150: 106272. DOI: 10.1016/j.mineng.2020.106272.
- [46] P. Huang, M. Cao, and Q. Liu, (2012) “Using chitosan as a selective depressant in the differential flotation of Cu– Pb sulfides" International Journal of Mineral Pro�cessing 106: 8–15. DOI: 10.1016/j.minpro.2012.01.001.