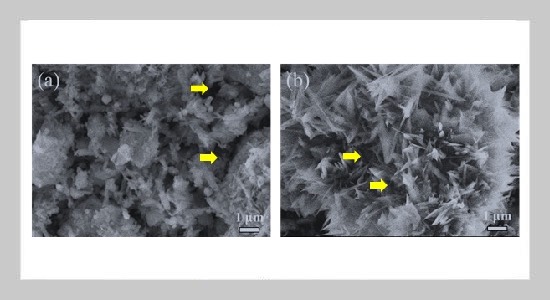
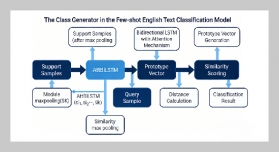
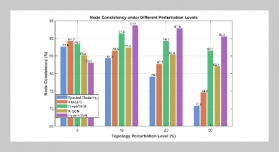
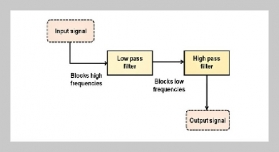
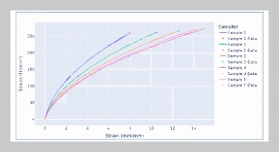
Tran Ngo Quan1,2 and Pham Trung Kien1,2,3,4This email address is being protected from spambots. You need JavaScript enabled to view it.
1Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet Street, HCMC, Vietnam
2Vietnam National University Ho Chi Minh City, Linh Trung Ward, , HCMC, Vietnam
3Polymer Research Center, HCMUT, 268 Ly Thuong Kiet Street, HCMC, Vietnam
4VNU-HCM Key Laboratorty for Medical Technologies, HCMUT, HCMC, Vietnam
- [1] A. Baldermann, A. Landler, F. Mittermayr, I. Letofsky-Papst, F. Steindl, I. Galan, and M. Dietzel, (2019) “Removal of heavy metals (Co, Cr, and Zn) dur ing calcium–aluminium–silicate–hydrate and trioctahedral smectite formation" Journal of Materials Science 54(13): 9331–9351.
- [2] M. A. Bhuiyan, M. Islam, S. B. Dampare, L. Parvez, and S. Suzuki, (2010) “Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh" Journal of hazardous materials 179(1-3): 1065–1077.
- [3] A. E. Burakov, E. V. Galunin, I. V. Burakova, A. E. Kucherova, S. Agarwal, A. G. Tkachev, and V. K. Gupta, (2018) “Adsorption of heavy metals on conventional and nanostructured materials for wastewater treat ment purposes: A review" Ecotoxicology and environ mental safety 148: 702–712.
- [4] L. Liu, S. Liu, H. Peng, Z. Yang, L. Zhao, and A. Tang, (2020) “Surface charge of mesoporous calcium silicate and its adsorption characteristics for heavy metal ions" Solid State Sciences 99: 106072.
- [5] J. A. Tsanakas, L. Ha, and C. Buerhop, (2016) “Faults and infrared thermographic diagnosis in operating c-Si photovoltaic modules: A review of research and future challenges" Renewable and sustainable energy reviews 62: 695–709.
- [6] C. Ballif, J. Dicker, D. Borchert, and T. Hofmann, (2004) “Solar glass with industrial porous SiO2 antireflection coating: measurements of photovoltaic module properties improvement and modelling of yearly energy yield gain" Solar energy materials and solar cells 82(3): 331–344.
- [7] P. Ramasamy, A. Periathamby, and S. Ibrahim, (2002) “Carbide sludge management in acetylene producing plants by using vacuum filtration" Waste management & re search 20(6): 536–540.
- [8] Z. Zhao, J. Wei, F. Li, X. Qu, L. Shi, H. Zhang, and Q. Yu, (2017) “Synthesis, characterization and hexavalent chromium adsorption characteristics of aluminum-and sucrose-incorporated tobermorite" Materials 10(6): 597.
- [9] C. Hejny and T. Armbruster, (2001) “Polytypism in xonotlite Ca6Si6O17 (OH) 2" Zeitschrift für Kristallographie-Crystalline Materials 216(7): 396–408.
- [10] P. Yu, R. J. Kirkpatrick, B. Poe, P. F. McMillan, and X. Cong, (1999) “Structure of calcium silicate hydrate (C-S H): Near-, Mid-, and Far-infrared spectroscopy" Journal of the American Ceramic Society 82(3): 742–748.
- [11] S. Steiner, B. Lothenbach, T. Proske, A. Borgschulte, and F. Winnefeld, (2020) “Effect of relative humidity on the carbonation rate of portlandite, calcium silicate hy drates and ettringite" Cement and Concrete Research 135: 106116.
- [12] M.Diez-Garcia, J. J. Gaitero, J. I. Santos, J. S. Dolado, and C. Aymonier, (2018) “Supercritical hydrothermal f low synthesis of xonotlite nanofibers" Journal of Flow Chemistry 8(2): 89–95.
- [13] Y. He, X. Zhao, L. Lu, L. J. Struble, and S. Hu, (2011) “Effect of C/S ratio on morphology and structure of hydrothermally synthesized calcium silicate hydrate" Journal of Wuhan University of Technology-Mater. Sci. Ed. 26(4): 770–773.
- [14] D. Luo, M. Xie, and X. Zhou. “Microwave assisted rapid synthesis of glycerol carbonate from glycerol catalyzed by anhydrous sodium silicate”. In: IOP Conference Series: Earth and Environmental Science. 687. 1. IOP Publishing. 2021, 012077.
- [15] N. D. Van, K. Imasawa, and Y. Hama, (2022) “Influence of hydrothermal synthesis conditions and carbonation on physical properties of xonotlite-based lightweight material" Construction and Building Materials 321: 126328.
- [16] L. Black, K. Garbev, and A. Stumm, (2009) “Structure, bonding and morphology of hydrothermally synthesised xonotlite" Advances in Applied Ceramics 108(3): 137–144.
- [17] Y. Li, W. Liu, F. Xing, S. Wang, L. Tang, S. Lin, and Z. Dong, (2020) “Carbonation of the synthetic calcium silicate hydrate (CSH) under different concentrations of CO2: Chemical phases analysis and kinetics" Journal of CO2Utilization 35: 303–313.
- [18] G. Zhu, H. Li, X. Wang, S. Li, X. Hou, W. Wu, and Q. Tang, (2016) “Synthesis of calcium silicate hydrate in highly alkaline system" Journal of the American Ceramic Society 99(8): 2778–2785.
- [19] Y. Yan, S.-Y. Yang, G. D. Miron, I. E. Collings, E. L’Hôpital, J. Skibsted, F. Winnefeld, K. Scrivener, and B. Lothenbach, (2022) “Effect of alkali hydroxide on calcium silicate hydrate (CSH)" Cement and Concrete Research 151: 106636.
- [20] C. Madi, M. Tabbal, T. Christidis, S. Isber, B. Nsouli, and K. Zahraman. “Microstructural characterization of chromium oxide thin films grown by remote plasma assisted pulsed laser deposition”. In: Journal of Physics: Conference Series. 59. 1. IOP Publishing. 2007, 600.
- [21] N. Annuar, S. Triwahyono, A. Jalil, N. Basar, T. Abdullah, and A. Ahmad, (2017) “Effect of Cr2O3 loading on the properties and cracking activity of Pt/Cr2O3 ZrO2" Applied Catalysis A: General 541: 77–86.
- [22] T. M. Al-Saadi and N. A. Hameed, (2015) “Synthesis and structural characterization of Cr2O3 nanoparticles prepared by using Cr (NO3)3.9H2Oandtriethanolamine undermicrowaveirr adiation" Synthesis 44:
- [23] E. Gevorkyan, L. Cepova, M. Rucki, V. Nerubatskyi, D. Morozow, W. Zurowski, V. Barsamyan, and K. Kouril, (2022) “Activated sintering of Cr2O3-based com posites by hot pressing" Materials 15(17): 5960.