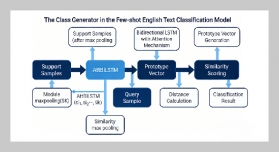
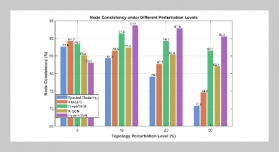
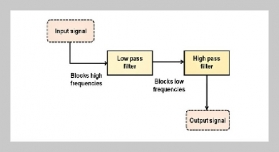
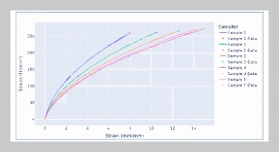
REFERENCES
- [1] Chiem, N. and Harrison, D. J., “Microchip-based Capillary Electrophoresis for Immunoassays: Analysis of Monoclonal Antibodies and Theophyllinne,” Anal. Chem., Vol. 69, pp. 373378 (1997).
- [2] Effenhauser, C. S., Bruin, G. J., Paulus, A., and Ehrat, M., “Integrated Capillary Electrophoresis on Flexible Silicone Microdevices: Analysis of DNA Restriction Fragments and Detection of Single DNA Molecules on Microchips,” Anal. Chem., Vol. 69, pp. 34513457 (1997).
- [3] Liu, Y., Foote, R. S., Culbertson, C. T., Jacobson, S. C., Ramsey, R. S. and Ramsey, J. M., “Electrophoretic Separation of Proteins on Microchips,” J. Microcolumn Separations, Vol. 12, pp. 407411 (2000).
- [4] Bharadwaj, R., Santiago, J. G. and Mohammadi, B., “Design and Optimization of On-chip Capillary Electrophoresis,” Electrophoresis, Vol. 23, pp. 27292744 (2002).
- [5] Carlier, J., Arscott, S., Thomy, V., Fourrier, J. C., Caron, F., Camart, J. C., Druon C., and Tabourier P., “Integrated Microfluidics Based on Multi-layered SU-8 for Mass Spectrometry Analysis,” J. Micromech. Microeng., Vol. 14, pp. 619624 (2004).
- [6] Majidiu, V., “Capillary Electrophoresis Inductively Coupled Plasma Mass Spectrometry,” Microchem. J., Vol. 66, pp. 316 (2000).
- [7] Lin, C. H., Lee, G. B., Chen S. H., and Chang, G. L., “Micro Capillary Electrophoresis Chips Integrated with Buried SU-8/SOG Optical Waveguides for Bio-analytical Applications,” Sensors and Actuators A, Vol. 107, pp. 125–131 (2003).
- [8] Cole, R. O., Hiller, D. L., Chwojdak, C. A., and Sepanidk, M. J., “Evaluation of Extended Light Path Capillaries for Use in Capillary Electrophoresis with Laser-induced Fluorescence Detection,” J. of Chromatography A, Vol. 736, pp. 239245 (1996).
- [9] Sinton, D., Ren, L., Xuan, X., and Li, D., “Effects of Liquid Conductivity Differences on Multi-component Sample Injection, Pumping and Stacking in Microfluidic Chips,” Lab Chip, Vol. 3, pp. 173–179 (2003).
- [10] Lloyd, D. K., “Capillary Electrophoretic Analyses of Drugs in Body Fluids: Sample Pretreatment and Methods for Direct Injection of Biofluids,” J. of Chromatography A, Vol. 735, pp. 2942 (1996).
- [11] Johnson1, M. E., and Landers, J. P., “Fundamentals and Practice for Ultrasensitive Laser-induced Fluorescence Detection in Microanalytical Systems,” Electrophoresis, Vol. 25, pp. 35133527 (2004).
- [12] Khandurian, J., Jacobson, S. C., Water, L. C., Foote, R. S., and Ramsey, J. M., “Microfabricated Porous Membrane Structure for Sample Concentration and Electrophoretic Analysis,” Anal. Chem., Vol. 71, pp. 1815 1819 (1999).
- [13] Lin, Y. C., Ho, H. C., Tseng, C. K., and Hou, S. Q., “A Poly-methylmethacrylate Electrophoresis Microchip with Sample Peconcentrator,” J. Micromech. Microeng., Vol. 11, pp. 189–194 (2000).
- [14] Ramos, A., Gonzalez, A., Castellanos, A., Green, N. G., and Morgan, H., “Pumping of Liquids with AC Voltages Applied to Asymmetric Pairs of Microelectrodes,” Physical review E, Vol. 7, pp. 111 (2003).
- [15] Lastochkin, D., Zhou, R., Wang, P., Ben, Y., and Chang, H. C., “Electrokinetic Micropump and Micromixer Design Based on AC Faradaic Polarization,” J. of applied physics, Vol. 96, pp. 1730���� �������
- [16] Morgan, H., Holmes, D., and Green, N. G., “3D Focusing of Nanoparticles in Microfluidics Channels,” IEE Proc.-Nanobiotechnol., Vol. 150, pp. 7680 (2003).
- [17] Oddy, M. H. and Santiago, J. G., “A Method for Determining Electrophoretic and Electroosmotic Mobilities Using AC and DC Electric Field Particle Displacements,” J. of Colloid and Interface Science, Vol. 269, pp. 192204 (2004).
- [18] Voldman, J., Braff, A. R., Toner, M., Gray, M. L., and Schmidt, M., “Holding Force of Single-Particle Dielectrophoretic Traps,” Biophysical J., Vol. 80, pp. 531541 (2001).
- [19] Khusid, B., Markarian, N., Yeksel, M., and Farmer, K. R., “Manipulation of Particle Motions and Their Segregation in Micro-Fluidics by Positive Dielectrophoresis,” International Mechanical Engineering Congress, Washington, D. C., Nov, 1521, p. 43008 (2003).
- [20] Zeng, S., Chen, C. H., Mikkelsen, J. C. Jr. and Santigo, J. G., “Fabrication and Characterization of Electroosmotic Micropumps,” Sensors and Actuators B, Vol. 79, pp. 107114 (2001).
- [21] Wang, P. K., Chen, C. Y., Wang, T. H., and Ho, C. M., “An AC Electroosmotic Processor for Biomolecules,” The 12th International Conference on Solid State Sensors, Actuators and Microsystems, Boston, June 812, pp. 2023 (2003).
- [22] Ramos, A., Morgan, H., Green, N. G. and Castellanosy, A., “AC Electrokinetics: A Review of Forces in Microelectrode Structures,” J. Phys. D: Appl. Phys., Vol. 31, pp. 23382353 (1998).
- [23] Castellanos, A., Ramos, A., Gonzalez, A., Morgan, H. and Green, N. G., “AC Electric-Field Fluid Flow in Microelectrode Structures: Scaling Laws,” Proceedings of 14th International Conference on Dielectric Liquids (ICDL 2002), Graz(Austria), July 712, pp. 5255 (2002).
- [24] Green, N. G., Ramos, A., Gonzalez, A., Morgan, H., and Castellanos, A., “Fluid Flow Induced by Nonuniform AC Electric Fields in Electrolytes on Microelectrodes. III. Observation of Streamlines and Numerical Simulation,”Physical Review E, Vol. 66, pp. 111 (2002).
- [25] Stroock, A. D., Weck, M., Chiu, D. T., Huck, W. T. S., and Kenis, P. J. A., “Patterning Electro-osmotic Folw with Patterned Surface Charge,” Physical Review Letters, Vol. 84, pp. 33143317 (2000).
- [26] Wong, P. K., Wang, T. H., Deval, J. H., and Ho, C. M., “Electrokinetics in Micro Devices for Biotechnology Applications,” IEEE/ASME transactions on mechatronics, Vol. 9, NO. 2, pp. 366376 (2004).
- [27] Liu, R. H., Vasile, M. J., and Beebe, D. J., “The Fabrication of Nonplanar Spin-On Glass Microstructures,” J. of Microelectromechanical Systems, Vol. 8, pp. 146151 (1999).
- [28] Chiang, C., and Fraser, D. B., “Understanding of Spinon-Glass (SOG) Properties from Their Molecular Structure,”VMIC IEEE, June 1213, pp. 397403 (1989).
- [29] Lin, C. H., Fu, L. M., Lee, K. H., Yang, R. J., and Lee, G. B., “Novel Surface Modification Methods and Surface Property Analysis for Separation of DNA Biomolecules Using Capillary Electrophoresis,” Submitted to the Seventh International Conference on Miniaturized Chemical and Biochemical Analysis Systems (-TAS), pp. 10811084 (2003).
- [30] Lee, G. B., Lin, C. H., Lee, C. Y., and Huang, F. C., “Microfluidic Chips for DNA Replication, Electrophoresis Separation and On-line Optical Detection” Proc. of the IEEE 16th International MEMS Conference, Kyoto, Japan, Jan. 1923, pp. 423426 (2003).
- [31] Lin, C. H., Lee, G. B., Lin, Y. H., and Chang, G. L., “A Fast Prototyping Process for Fabrication of Microfluidic Systems on Soda-lime Glass,” J. of Micromechanics and Microengineering, Vol. 11, pp. 726732 (2001).
- [32] Green, N. G, Ramos, A., Gonzalez, A., Morgan, H., and Castellanos, A., “Fluid Flow Induced by Nonuniform AC Electric Fields in Electrolytes on Microelectrodes. I. Experimental measurements,” Physical Review E, Vol. 61, pp. 40114018 (2000).